Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines
- PMID: 23300088
- PMCID: PMC3585078
- DOI: 10.1074/jbc.M112.412478
Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines
Abstract
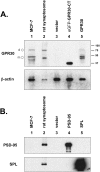
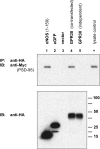
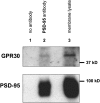
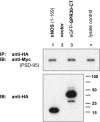
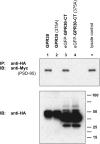
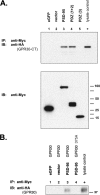
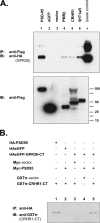
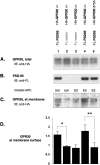
The estrogen 17β-estradiol (E2) modulates dendritic spine plasticity in the cornu ammonis 1 (CA1) region of the hippocampus, and GPR30 (G-protein coupled estrogen receptor 1 (GPER1)) is an estrogen-sensitive G-protein-coupled receptor (GPCR) that is expressed in the mammalian brain and in specific subregions that are responsive to E2, including the hippocampus. The subcellular localization of hippocampal GPR30, however, remains unclear. Here, we demonstrate that GPR30 immunoreactivity is detected in dendritic spines of rat CA1 hippocampal neurons in vivo and that GPR30 protein can be found in rat brain synaptosomes. GPR30 immunoreactivity is identified at the post-synaptic density (PSD) and in the adjacent peri-synaptic zone, and GPR30 can associate with the spine scaffolding protein PSD-95 both in vitro and in vivo. This PSD-95 binding capacity of GPR30 is specific and determined by the receptor C-terminal tail that is both necessary and sufficient for PSD-95 interaction. The interaction with PSD-95 functions to increase GPR30 protein levels residing at the plasma membrane surface. GPR30 associates with the N-terminal tandem pair of PDZ domains in PSD-95, suggesting that PSD-95 may be involved in clustering GPR30 with other receptors in the hippocampus. We demonstrate that GPR30 has the potential to associate with additional post-synaptic GPCRs, including the membrane progestin receptor, the corticotropin releasing hormone receptor, and the 5HT1a serotonin receptor. These data demonstrate that GPR30 is well positioned in the dendritic spine compartment to integrate E2 sensitivity directly onto multiple inputs on synaptic activity and might begin to provide a molecular explanation as to how E2 modulates dendritic spine plasticity.
Figures
References
-
- McEwen B. S. (2001) Invited review. Estrogens effects on the brain. Multiple sites and molecular mechanisms. J. Appl. Physiol. 91, 2785–2801 - PubMed
-
- Thomas P., Pang Y., Filardo E. J., Dong J. (2005) Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 - PubMed
-
- Carmeci C., Thompson D. A., Ring H. Z., Francke U., Weigel R. J. (1997) Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 45, 607–617 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

