Horizontal gene transfer contributed to the evolution of extracellular surface structures: the freshwater polyp Hydra is covered by a complex fibrous cuticle containing glycosaminoglycans and proteins of the PPOD and SWT (sweet tooth) families
- PMID: 23300632
- PMCID: PMC3531485
- DOI: 10.1371/journal.pone.0052278
Horizontal gene transfer contributed to the evolution of extracellular surface structures: the freshwater polyp Hydra is covered by a complex fibrous cuticle containing glycosaminoglycans and proteins of the PPOD and SWT (sweet tooth) families
Abstract
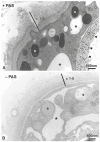
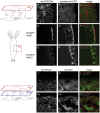
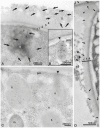
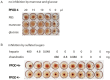
The single-cell layered ectoderm of the fresh water polyp Hydra fulfills the function of an epidermis by protecting the animals from the surrounding medium. Its outer surface is covered by a fibrous structure termed the cuticle layer, with similarity to the extracellular surface coats of mammalian epithelia. In this paper we have identified molecular components of the cuticle. We show that its outermost layer contains glycoproteins and glycosaminoglycans and we have identified chondroitin and chondroitin-6-sulfate chains. In a search for proteins that could be involved in organising this structure we found PPOD proteins and several members of a protein family containing only SWT (sweet tooth) domains. Structural analyses indicate that PPODs consist of two tandem β-trefoil domains with similarity to carbohydrate-binding sites found in lectins. Experimental evidence confirmed that PPODs can bind sulfated glycans and are secreted into the cuticle layer from granules localized under the apical surface of the ectodermal epithelial cells. PPODs are taxon-specific proteins which appear to have entered the Hydra genome by horizontal gene transfer from bacteria. Their acquisition at the time Hydra evolved from a marine ancestor may have been critical for the transition to the freshwater environment.
Conflict of interest statement
Figures






References
-
- Sarras MP, Deutzmann R (2001) Hydra and Niccolo Paganini (1782–1840)–two peas in a pod? The molecular basis of extracellular matrix structure in the invertebrate, Hydra . Bioessays 23: 716–724. - PubMed
-
- Lentz TL (1966) The Cell Biology of Hydra. Amsterdam: North-Holland publishing company. 199 p.
-
- Holtmann M, Thurm U (2001) Variations of concentric hair cells in a Cnidarian sensory epithelium (Coryne tubulosa). J Comp Neurol 432: 550–563. - PubMed
-
- Thomas MB, Edwards NC, Higgins RP (1995) Cryptohydra thieli n.gen., sp.: a meiofaunal marine hydroid (Hydroida, Athecata, Capitata). Invertebrate Biology 114: 107–118.
-
- Kuznetsov SG, Anton-Erxleben F, Bosch TC (2002) Epithelial interactions in Hydra: apoptosis in interspecies grafts is induced by detachment from the extracellular matrix. J Exp Biol 205: 3809–3817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

