IL-13 promotes collagen accumulation in Crohn's disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells?
- PMID: 23300643
- PMCID: PMC3534115
- DOI: 10.1371/journal.pone.0052332
IL-13 promotes collagen accumulation in Crohn's disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells?
Abstract
Background: Fibrosis is a serious consequence of Crohn's disease (CD), often necessitating surgical resection. We examined the hypothesis that IL-13 may promote collagen accumulation within the CD muscle microenvironment.
Methods: Factors potentially modulating collagen deposition were examined in intestinal tissue samples from fibrotic (f) CD and compared with cancer control (C), ulcerative colitis (UC) and uninvolved (u) CD. Mechanisms attributable to IL-13 were analysed using cell lines derived from uninvolved muscle tissue and tissue explants.
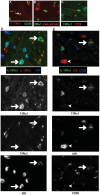
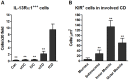
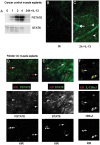
Results: In fCD muscle extracts, collagen synthesis was significantly increased compared to other groups, but MMP-2 was not co-ordinately increased. IL-13 transcripts were highest in fCD muscle compared to muscle from other groups. IL-13 receptor (R) α1 was expressed by intestinal muscle smooth muscle, nerve and KIR(+) cells. Fibroblasts from intestinal muscle expressed Rα1, phosphorylated STAT6 in response to IL-13, and subsequently down-regulated MMP-2 and TNF-α-induced MMP-1 and MMP-9 synthesis. Cells with the phenotype KIR(+)CD45(+)CD56(+/-)CD3(-) were significantly increased in fCD muscle compared to all other groups, expressed Rα1 and membrane IL-13, and transcribed high levels of IL-13. In explanted CD muscle, these cells did not phosphorylate STAT6 in response to exogenous IL-13.
Conclusions: The data indicate that in fibrotic intestinal muscle of Crohn's patients, the IL-13 pathway is stimulated, involving a novel population of infiltrating IL-13Rα1(+), KIR(+) innate lymphoid cells, producing IL-13 which inhibits fibroblast MMP synthesis. Consequently, matrix degradation is down-regulated and this leads to excessive collagen deposition.
Conflict of interest statement
Figures






References
-
- Burke JP, Mulsow JJ, O’Keane C, Docherty NG, Watson RW, et al. (2007) Fibrogenesis in Crohn’s disease. Am J Gastroenterol 102: 439–448. - PubMed
-
- Mourelle M, Salas A, Guarner F, Crespo E, Garcia-Lafuente A, et al. (1998) Stimulation of transforming growth factor beta1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology 114: 519–526. - PubMed
-
- Wynn TA (2003) IL-13 effector functions. Annu Rev Immunol 21: 425–456. - PubMed
-
- Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, et al. (2000) Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther 292: 988–994. - PubMed
-
- Koukoulis G, Ke Y, Henley JD, Cummings OW (2001) Obliterative muscularization of the small bowel submucosa in Crohn disease: a possible mechanism of small bowel obstruction. Arch Pathol Lab Med 125: 1331–1334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

