Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture
- PMID: 23300850
- PMCID: PMC3534089
- DOI: 10.1371/journal.pone.0053024
Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture
Abstract
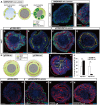
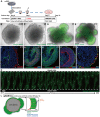
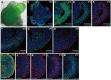
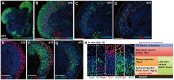
In the mammalian cortex, the dorsal telencephalon exhibits a characteristic stratified structure. We previously reported that three-dimensional (3D) culture of mouse ES cells (mESCs) can efficiently generate cortical neuroepithelium (NE) and layer-specific cortical neurons. However, the cortical NE generated in this mESC culture was structurally unstable and broke into small neural rosettes by culture day 7, suggesting that some factors for reinforcing the structural integrity were missing. Here we report substantial supporting effects of the extracellular matrix (ECM) protein laminin on the continuous formation of properly polarized cortical NE in floating aggregate culture of mESCs. The addition of purified laminin and entactin (a laminin-associated protein), even at low concentrations, stabilized the formation of continuous cortical NE as well as the maintenance of basement membrane and prevented rosette formation. Treatment with the neutralizing ß1-integrin antibody impaired the continuous NE formation. The stabilized cortical NE exhibited typical interkinetic nuclear migration of cortical progenitors, as seen in the embryonic cortex. The laminin-treated cortical NE maintained a continuous structure even on culture days 12 and 15, and contained ventricular, basal-progenitor, cortical-plate and Cajal-Retzius cell layers. The cortical NE in this culture was flanked by cortical hem-like tissue. Furthermore, when Shh was added, ventral telencephalic structures such as lateral ganglionic eminence-like tissue formed in the region adjacent to the cortical NE. Thus, our results indicate that laminin-entactin ECM promotes the formation of structurally stable telencephalic tissues in 3D ESC culture, and supports the morphogenetic recapitulation of cortical development.
Conflict of interest statement
Figures
References
-
- Rallu M, Corbin JG, Fishell G (2002) Parsing the prosencephalon. Nature Rev Neurosci 3: 943–951. - PubMed
-
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL (1995) Longitudinal organization of the anterior neural plate and neural tube. Development 121: 3923–3933. - PubMed
-
- Hevner RF, Daza RAM, Rubenstein JLR, Stunnenberg H, Olavarria JF, et al. (2003) Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev Neurosci 25: 139–151. - PubMed
-
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, et al. (2006) The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nature neurosci 9: 743–751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

