Defective soil for a fertile seed? Altered endometrial development is detrimental to pregnancy success
- PMID: 23300868
- PMCID: PMC3533948
- DOI: 10.1371/journal.pone.0053098
Defective soil for a fertile seed? Altered endometrial development is detrimental to pregnancy success
Abstract
Background: Synchronous development of the endometrium (to achieve a receptive state) and of the embryo is essential for successful implantation and ongoing pregnancy. Endometrial receptivity exists only for a finite time in a menstrual cycle and the endometrium is refractory to embryo implantation outside of this window. Administration of hormones to stimulate multifollicular development within the ovary, integral to the majority of assisted reproduction (ART) protocols, dramatically alters the hormonal milieu to which the endometrium is exposed versus normal menstrual cycles. Endometrial maturation may be profoundly affected by this altered endocrine environment.
Aim: Compare endometrial histology in fertile women, fertile women undergoing hormonal stimulation for oocyte donation and infertile women undergoing fresh embryo transfers in an ART cycle with further comparisons between women who did or did not become pregnant. Examine the presence of leukocytes and markers of endometrial maturation.
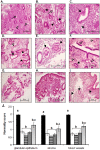
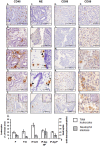
Methods: Endometrial histology was examined by hematoxylin and eosin staining with a semi quantitative scoring method developed to compare histological appearance of tissues. The presence of leukocytes and developmental markers was examined by immunohistochemistry and scored.
Results: Endometrial histology was dramatically altered upon stimulation for ART. However, those women who became pregnant presented with significantly less alterations in histological endometrial maturation. Numbers and activation status of leukocyte populations were also altered within the endometria stimulated for ART, with neutrophils undergoing degranulation, usually observed only pre-menstrually.
Conclusion: We propose that such developmental changes render the endometrium hostile to the embryo and that modifications to ART protocols should be considered to take account of the requirement for endometrial receptivity and hence increase pregnancy rates.
Conflict of interest statement
Figures


References
-
- Salamonsen LA, Nie G, Hannan NJ, Dimitriadis E (2009) Society for Reproductive Biology Founders' Lecture 2009. Preparing fertile soil: the importance of endometrial receptivity. Reprod Fertil Dev 21: 923–934. - PubMed
-
- van der Gaast MH, Classen-Linke I, Krusche CA, Beier-Hellwig K, Fauser BC, et al. (2008) Impact of ovarian stimulation on mid-luteal endometrial tissue and secretion markers of receptivity. Reprod Biomed Online 17: 553–563. - PubMed
-
- Boomsma CM, Kavelaars A, Eijkemans MJ, Amarouchi K, Teklenburg G, et al. (2009) Cytokine profiling in endometrial secretions: a non-invasive window on endometrial receptivity. Reprod Biomed Online 18: 85–94. - PubMed
-
- Boomsma CM, Kavelaars A, Eijkemans MJ, Lentjes EG, Fauser BC, et al. (2009) Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum Reprod 24: 1427–1435. - PubMed
-
- Boomsma CM, Kavelaars A, Eijkemans MJ, Fauser BC, Heijnen CJ, et al. (2010) Ovarian stimulation for in vitro fertilization alters the intrauterine cytokine, chemokine, and growth factor milieu encountered by the embryo. Fertil Steril 94: 1764–1768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

