Visualising the cross-level relationships between pathological and physiological processes and gene expression: analyses of haematological diseases
- PMID: 23301083
- PMCID: PMC3534650
- DOI: 10.1371/journal.pone.0053544
Visualising the cross-level relationships between pathological and physiological processes and gene expression: analyses of haematological diseases
Abstract
The understanding of pathological processes is based on the comparison between physiological and pathological conditions, and transcriptomic analysis has been extensively applied to various diseases for this purpose. However, the way in which the transcriptomic data of pathological cells relate to the transcriptomes of normal cellular counterparts has not been fully explored, and may provide new and unbiased insights into the mechanisms of these diseases. To achieve this, it is necessary to develop a method to simultaneously analyse components across different levels, namely genes, normal cells, and diseases. Here we propose a multidimensional method that visualises the cross-level relationships between these components at three different levels based on transcriptomic data of physiological and pathological processes, by adapting Canonical Correspondence Analysis, which was developed in ecology and sociology, to microarray data (CCA on Microarray data, CCAM). Using CCAM, we have analysed transcriptomes of haematological disorders and those of normal haematopoietic cell differentiation. First, by analysing leukaemia data, CCAM successfully visualised known relationships between leukaemia subtypes and cellular differentiation, and their characteristic genes, which confirmed the relevance of CCAM. Next, by analysing transcriptomes of myelodysplastic syndromes (MDS), we have shown that CCAM was effective in both generating and testing hypotheses. CCAM showed that among MDS patients, high-risk patients had transcriptomes that were more similar to those of both haematopoietic stem cells (HSC) and megakaryocyte-erythroid progenitors (MEP) than low-risk patients, and provided a prognostic model. Collectively, CCAM reveals hidden relationships between pathological and physiological processes and gene expression, providing meaningful clinical insights into haematological diseases, and these could not be revealed by other univariate and multivariate methods. Furthermore, CCAM was effective in identifying candidate genes that are correlated with cellular phenotypes of interest. We expect that CCAM will benefit a wide range of medical fields.
Conflict of interest statement
Figures


 cells from MDS, non-MDS anaemia, and healthy controls were analysed by CCAM using five haematopoietic cell populations (Haematopoitic stem cell [HSC], Megakaryocyte-erythroid progenitors [MEP], Common myeloid progenitor [CMP], Granulocyte-monocyte progenitor [GMP], Pro-B cell) as explanatory variables. Genes were filtered by MDS data using an empirical Bayes t-statistic [
cells from MDS, non-MDS anaemia, and healthy controls were analysed by CCAM using five haematopoietic cell populations (Haematopoitic stem cell [HSC], Megakaryocyte-erythroid progenitors [MEP], Common myeloid progenitor [CMP], Granulocyte-monocyte progenitor [GMP], Pro-B cell) as explanatory variables. Genes were filtered by MDS data using an empirical Bayes t-statistic [ ]). (a) CCA triplot. Centroids of MDS and normal CD34
]). (a) CCA triplot. Centroids of MDS and normal CD34 cells are shown by large closed circles, and 95% confident intervals (CI) are indicated by ellipsoids. Genes are shown by closed grey circles, and well-known genes that are key for either MDS or corresponding haematopoietic cells are annotated. Axis 1 indicates the direction to which the variation (inertia) is the largest (87% of the total variation [constrained inertia]). Axis 2 has the second largest inertia (7%). (b) Individual disease samples are shown without genes to clearly show the relationships between disease samples and haematopoietic cell populations.
cells are shown by large closed circles, and 95% confident intervals (CI) are indicated by ellipsoids. Genes are shown by closed grey circles, and well-known genes that are key for either MDS or corresponding haematopoietic cells are annotated. Axis 1 indicates the direction to which the variation (inertia) is the largest (87% of the total variation [constrained inertia]). Axis 2 has the second largest inertia (7%). (b) Individual disease samples are shown without genes to clearly show the relationships between disease samples and haematopoietic cell populations.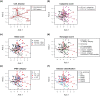
 ), therefore this is an unsupervised analysis in terms of MDS disease data. (a) CCAM result showing the disease and cell levels. Axis 1 indicates the direction to which the variation (inertia) is the largest (55% of the total variation [constrained inertia]). Axis 2 has the second largest inertia (21%). MDS patient samples are positioned according to the correlations with five haematopoietic cell populations in terms of gene expression. (b–d) The following clinical data of individual disease samples were superimposed on the map in (a): (b) cytopenia score; (c) blast score; (d) karyotype score; (e) IPSS category; and (f) disease classification.
), therefore this is an unsupervised analysis in terms of MDS disease data. (a) CCAM result showing the disease and cell levels. Axis 1 indicates the direction to which the variation (inertia) is the largest (55% of the total variation [constrained inertia]). Axis 2 has the second largest inertia (21%). MDS patient samples are positioned according to the correlations with five haematopoietic cell populations in terms of gene expression. (b–d) The following clinical data of individual disease samples were superimposed on the map in (a): (b) cytopenia score; (c) blast score; (d) karyotype score; (e) IPSS category; and (f) disease classification.
 and 2:
and 2:  ). (b) HSC-CMP score, three groups (1:
). (b) HSC-CMP score, three groups (1:  , 2:
, 2:  , and 3:
, and 3:  ). (c) Cytopenia score. (d) Blast score. (e) Karyotype score. (f) IPSS score. (g) Disease classification. P values are by log-rank test.
). (c) Cytopenia score. (d) Blast score. (e) Karyotype score. (f) IPSS score. (g) Disease classification. P values are by log-rank test.
 and 2:
and 2:  ). (b) HSC-CMP score, three groups (1:
). (b) HSC-CMP score, three groups (1:  , 2:
, 2:  , and 3:
, and 3:  ). (c) Cytopenia score. (d) Blast score. (e) Karyotype score. (f) IPSS score. (g) Disease classification. P values are by log-rank test.
). (c) Cytopenia score. (d) Blast score. (e) Karyotype score. (f) IPSS score. (g) Disease classification. P values are by log-rank test.
References
-
- Liu R, Wang X, Chen GY, Dalerba P, Gurney A, et al. (2007) The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 356: 217–26. - PubMed
-
- van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, et al. (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415: 530–6. - PubMed
-
- Greenberg SA, Higgs BW, Morehouse C, Walsh RJ, Won Kong S, et al. (2012) Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun 13: 207–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

