Field evaluation and impact on clinical management of a rapid diagnostic kit that detects dengue NS1, IgM and IgG
- PMID: 23301110
- PMCID: PMC3531494
- DOI: 10.1371/journal.pntd.0001993
Field evaluation and impact on clinical management of a rapid diagnostic kit that detects dengue NS1, IgM and IgG
Abstract
Background: Dengue diagnosis is complex and until recently only specialized laboratories were able to definitively confirm dengue infection. Rapid tests are now available commercially making biological diagnosis possible in the field. The aim of this study was to evaluate a combined dengue rapid test for the detection of NS1 and IgM/IgG antibodies. The evaluation was made prospectively in the field conditions and included the study of the impact of its use as a point-of-care test for case management as well as retrospectively against a panel of well-characterized samples in a reference laboratory.
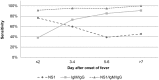
Methodology/principal findings: During the prospective study, 157 patients hospitalized for a suspicion of dengue were enrolled. In the hospital laboratories, the overall sensitivity, specificity, PPV and NPV of the NS1/IgM/IgG combination tests were 85.7%, 83.9%, 95.6% and 59.1% respectively, whereas they were 94,4%, 90.0%, 97.5% and 77.1% respectively in the national reference laboratory at Institut Pasteur in Cambodia. These results demonstrate that optimal performances require adequate training and quality assurance. The retrospective study showed that the sensitivity of the combined kit did not vary significantly between the serotypes and was not affected by the immune status or by the interval of time between onset of fever and sample collection. The analysis of the medical records indicates that the physicians did not take into consideration the results obtained with the rapid test including for care management and use of antibiotic therapy.
Conclusions: In the context of our prospective field study, we demonstrated that if the SD Bioline Dengue Duo kit is correctly used, a positive result highly suggests a dengue case but a negative result doesn't rule out a dengue infection. Nevertheless, Cambodian pediatricians in their daily practice relied on their clinical diagnosis and thus the false negative results obtained did not directly impact on the clinical management.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The diagnostic sensitivity of dengue rapid test assays is significantly enhanced by using a combined antigen and antibody testing approach.PLoS Negl Trop Dis. 2011 Jun;5(6):e1199. doi: 10.1371/journal.pntd.0001199. Epub 2011 Jun 21. PLoS Negl Trop Dis. 2011. PMID: 21713023 Free PMC article.
-
Assessment of diagnostic and analytic performance of the SD Bioline Dengue Duo test for dengue virus (DENV) infections in an endemic area (Savannakhet province, Lao People's Democratic Republic).PLoS One. 2020 Mar 17;15(3):e0230337. doi: 10.1371/journal.pone.0230337. eCollection 2020. PLoS One. 2020. PMID: 32182271 Free PMC article.
-
EVALUATION OF A COMMERCIAL RAPID TEST KIT FOR DETECTION OF ACUTE DENGUE INFECTION.Southeast Asian J Trop Med Public Health. 2015 Jul;46(4):602-10. Southeast Asian J Trop Med Public Health. 2015. PMID: 26867379
-
Systematic review and meta-analysis: assessing the accuracy of rapid immunochromatographic tests in dengue diagnosis.Diagn Microbiol Infect Dis. 2024 Jun;109(2):116227. doi: 10.1016/j.diagmicrobio.2024.116227. Epub 2024 Feb 20. Diagn Microbiol Infect Dis. 2024. PMID: 38503028
-
A systematic review and meta-analysis on the accuracy of rapid immunochromatographic tests for dengue diagnosis.Eur J Clin Microbiol Infect Dis. 2022 Sep;41(9):1191-1201. doi: 10.1007/s10096-022-04485-6. Epub 2022 Aug 20. Eur J Clin Microbiol Infect Dis. 2022. PMID: 35988010
Cited by
-
Use of a Rapid Test for Diagnosis of Dengue during Suspected Dengue Outbreaks in Resource-Limited Regions.J Clin Microbiol. 2016 Aug;54(8):2090-5. doi: 10.1128/JCM.00521-16. Epub 2016 May 25. J Clin Microbiol. 2016. PMID: 27225409 Free PMC article.
-
Evaluating dengue burden in Africa in passive fever surveillance and seroprevalence studies: protocol of field studies of the Dengue Vaccine Initiative.BMJ Open. 2018 Jan 21;8(1):e017673. doi: 10.1136/bmjopen-2017-017673. BMJ Open. 2018. PMID: 29358421 Free PMC article.
-
Spatial epidemiology and climatic predictors of paediatric dengue infections captured via sentinel site surveillance, Phnom Penh Cambodia 2011-2012.BMC Public Health. 2014 Jun 28;14:658. doi: 10.1186/1471-2458-14-658. BMC Public Health. 2014. PMID: 24972712 Free PMC article.
-
Diagnostic Options and Challenges for Dengue and Chikungunya Viruses.Biomed Res Int. 2015;2015:834371. doi: 10.1155/2015/834371. Epub 2015 Oct 5. Biomed Res Int. 2015. PMID: 26509163 Free PMC article. Review.
-
Evaluation of rapid diagnostic tests to detect dengue virus infections in Taiwan.PLoS One. 2020 Sep 29;15(9):e0239710. doi: 10.1371/journal.pone.0239710. eCollection 2020. PLoS One. 2020. PMID: 32991592 Free PMC article.
References
-
- WHO/TDR (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. 3rd edition. - PubMed
-
- Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, et al. (2002) Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol 40: 376–381. - PMC - PubMed