Human-induced marine ecological degradation: micropaleontological perspectives
- PMID: 23301187
- PMCID: PMC3539015
- DOI: 10.1002/ece3.425
Human-induced marine ecological degradation: micropaleontological perspectives
Abstract
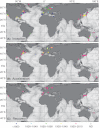
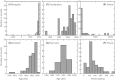
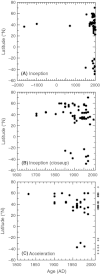
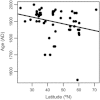
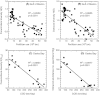
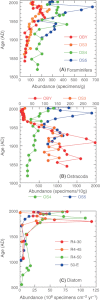
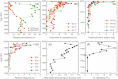
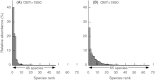
We analyzed published downcore microfossil records from 150 studies and reinterpreted them from an ecological degradation perspective to address the following critical but still imperfectly answered questions: (1) How is the timing of human-induced degradation of marine ecosystems different among regions? (2) What are the dominant causes of human-induced marine ecological degradation? (3) How can we better document natural variability and thereby avoid the problem of shifting baselines of comparison as degradation progresses over time? The results indicated that: (1) ecological degradation in marine systems began significantly earlier in Europe and North America (∼1800s) compared with Asia (post-1900) due to earlier industrialization in European and North American countries, (2) ecological degradation accelerated globally in the late 20th century due to post-World War II economic growth, (3) recovery from the degraded state in late 20th century following various restoration efforts and environmental regulations occurred only in limited localities. Although complex in detail, typical signs of ecological degradation were diversity decline, dramatic changes in total abundance, decrease in benthic and/or sensitive species, and increase in planktic, resistant, toxic, and/or introduced species. The predominant cause of degradation detected in these microfossil records was nutrient enrichment and the resulting symptoms of eutrophication, including hypoxia. Other causes also played considerable roles in some areas, including severe metal pollution around mining sites, water acidification by acidic wastewater, and salinity changes from construction of causeways, dikes, and channels, deforestation, and land clearance. Microfossils enable reconstruction of the ecological history of the past 10(2)-10(3) years or even more, and, in conjunction with statistical modeling approaches using independent proxy records of climate and human-induced environmental changes, future research will enable workers to better address Shifting Baseline Syndrome and separate anthropogenic impacts from background natural variability.
Keywords: Eutrophication; hypoxia; marine ecosystems; microfossils; pollution; species diversity.
Figures




References
-
- Abe K. Population structure of Keijella bisanensis (Okubo) (Ostracoda, Crustacea) – An inquiry into how far the population structure will be preserved in the fossil record. J. Fac. Sci., Univ. Tokyo. Sec. II, Geol. Mineral. Geogr. Geophys. 1983;20:443–488.
-
- Airoldi L, Beck MW. Loss, status and trends for coastal marine habitat of Europe. Oceanogr. Mar. Biol. Annu. Rev. 2007;45:345–405.
-
- Akimoto K, Nakamura K, Kondo H, Ishiga H, Dozen K. Environmental reconstruction based on heavy metals, diatoms and benthic foraminifes in the Isahaya reclamation area, Nagasaki, Japan. Environ. Micropaleontol., Microbiol. and Meiobenthol. 2004;1:83–104.
-
- Allen AP, Brown JH, Gillooly JF. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science. 2002;297:1545–1548. - PubMed
-
- Alvarez Zarikian CA, Blackwelder PL, Hood T, Nelsen TA, Featherstone C. 2000. pp. 896–905. Ostracods as indicators of natural and anthropogenically-induced changes in coastal marine environments. Coasts at the Millennium, Proceedings of the 17th International Conference of The Coastal Society, Portland, OR USA, July 9-12.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

