Nuclear expression of PG-21, SRC-1, and pCREB in regions of the lumbosacral spinal cord involved in pelvic innervation in young adult and aged rats
- PMID: 23301192
- PMCID: PMC3531588
- DOI: 10.5115/acb.2012.45.4.241
Nuclear expression of PG-21, SRC-1, and pCREB in regions of the lumbosacral spinal cord involved in pelvic innervation in young adult and aged rats
Abstract
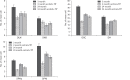
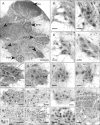
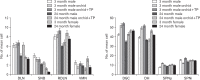
In rats, ageing results in dysfunctional patterns of micturition and diminished sexual reflexes that may reflect degenerative changes within spinal circuitry. In both sexes the dorsal lateral nucleus and the spinal nucleus of the bulbospongiosus, which lie in the L5-S1 spinal segments, contain motor neurons that innervate perineal muscles, and the external anal and urethral sphincters. Neurons in the sacral parasympathetic nucleus of these segments provide autonomic control of the bladder, cervix and penis and other lower urinary tract structures. Interneurons in the dorsal gray commissure and dorsal horn have also been implicated in lower urinary tract function. This study investigates the cellular localisation of PG-21 androgen receptors, steroid receptor co-activator one (SRC-1) and the phosphorylated form of c-AMP response element binding protein (pCREB) within these spinal nuclei. These are components of signalling pathways that mediate cellular responses to steroid hormones and neurotrophins. Nuclear expression of PG-21 androgen receptors, SRC-1 and pCREB in young and aged rats was quantified using immunohistochemistry. There was a reduction in the number of spinal neurons expressing these molecules in the aged males while in aged females, SRC-1 and pCREB expression was largely unchanged. This suggests that the observed age-related changes may be linked to declining testosterone levels. Acute testosterone therapy restored expression of PG-21 androgen receptor in aged and orchidectomised male rats, however levels of re-expression varied within different nuclei suggesting a more prolonged period of hormone replacement may be required for full restoration.
Keywords: Androgens; Hormone replacement therapy; Pelvic function; Perineal motor neuron.
Figures


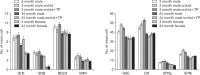

References
-
- Dering MA, Santer RM, Watson AH. Age-related changes in the morphology of preganglionic neurons projecting to the rat hypogastric ganglion. J Neurocytol. 1996;25:555–563. - PubMed
-
- Hancock MB, Peveto CA. Preganglionic neurons in the sacral spinal cord of the rat: an HRP study. Neurosci Lett. 1979;11:1–5. - PubMed
-
- Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984;226:238–245. - PubMed
-
- Ranson RN, Santer RM, Watson AH. The relationship between serotonin, dopamine beta hydroxylase and GABA immunoreactive inputs and spinal preganglionic neurones projecting to the major pelvic ganglion of Wistar rats. Neuroscience. 2006;141:1935–1949. - PubMed
-
- Santer RM, Dering MA, Ranson RN, Waboso HN, Watson AH. Differential susceptibility to ageing of rat preganglionic neurones projecting to the major pelvic ganglion and of their afferent inputs. Auton Neurosci. 2002;96:73–81. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
