Behavioral and histopathological alterations resulting from mild fluid percussion injury
- PMID: 23301501
- PMCID: PMC3941923
- DOI: 10.1089/neu.2012.2630
Behavioral and histopathological alterations resulting from mild fluid percussion injury
Abstract
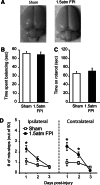
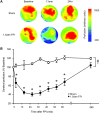
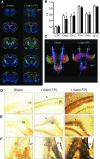
The majority of people who sustain a traumatic brain injury (TBI) have an injury that can be classified as mild (often referred to as concussion). Although head CT scans for most subjects who have sustained a mild TBI (mTBI) are negative, these persons may still suffer from neurocognitive and neurobehavioral deficits. In order to expedite pre-clinical research and develop therapies, there is a need for well-characterized animal models of mTBI that reflect the neurological, neurocognitive, and pathological changes seen in human patients. In the present study, we examined the motor, cognitive, and histopathological changes resulting from 1.0 and 1.5 atmosphere (atm) overpressure fluid percussion injury (FPI). Both 1.0 and 1.5 atm FPI injury caused transient suppression of acute neurological functions, but did not result in visible brain contusion. Animals injured with 1.0 atm FPI did not show significant motor, vestibulomotor, or learning and memory deficits. In contrast, 1.5 atm injury caused transient motor disturbances, and resulted in a significant impairment of spatial learning and short-term memory. In addition, 1.5 atm FPI caused a marked reduction in cerebral perfusion at the site of injury that lasted for several hours. Consistent with previous studies, 1.5 atm FPI did not cause visible neuronal loss in the hippocampus or in the neocortex. However, a robust inflammatory response (as indicated by enhanced GFAP and Iba1 immunoreactivity) in the corpus callosum and the thalamus was observed. Examination of fractional anisotropy color maps after diffusion tensor imaging (DTI) revealed a significant decrease of FA values in the cingulum, an area found to have increased silver impregnation, suggesting axonal injury. Increased silver impregnation was also observed in the corpus callosum, and internal and external capsules. These findings are consistent with the deficits and pathologies associated with mild TBI in humans, and support the use of mild FPI as a model to evaluate putative therapeutic options.
Figures






References
-
- Alexander MP. Mild traumatic brain injury: Pathophysiology, natural history, and clinical management. Neurology. 1995;45:1253–1260. - PubMed
-
- Binder LM. A review of mild head trauma. Part II: Clinical implications. J Clin Exp Neuropsychol. 1997;19:432–457. - PubMed
-
- d'Hemecourt P. Subacute symptoms of sports-related concussion: Outpatient management and return to play. Clin Sports Med. 2011;30:63–72. - PubMed
-
- Ruff RM. Camenzuli L. Mueller J. Miserable minority: Emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj. 1996;10:551–565. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

