Multiple pluripotent stem cell markers in human anaplastic thyroid cancer: the putative upstream role of SOX2
- PMID: 23301671
- PMCID: PMC3704189
- DOI: 10.1089/thy.2012.0372
Multiple pluripotent stem cell markers in human anaplastic thyroid cancer: the putative upstream role of SOX2
Abstract
Background: Anaplastic thyroid carcinoma (ATC) is a rare and aggressive endocrine tumor with highly undifferentiated morphology. It has been suggested that cancer stem cells (CSCs) might play a central role in ATC. The objectives of this study were (i) to characterize CSCs from ex vivo ATC specimens by investigating the expression of several pluripotent stem cell markers, and (ii) to evaluate in vitro drug resistance modifications after specific CSC transcription factor switch-off.
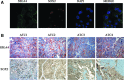
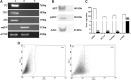
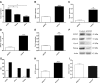
Methods: In ex vivo experiments, eight formalin-fixed, paraffin-embedded ATC specimens were analyzed by reverse-transcription and real-time quantitative PCR and immunohistochemistry. In in vitro experiments using ATC SW1736 cells, the expression levels of OCT-4, NANOG, and ABCG2 and the sensitivity to either cisplatin or doxorubicin were evaluated after silencing.
Results: OCT-4, KLF4, and SOX2 transcription factors and C-KIT and THY-1 stem surface antigens showed variable up-regulation in all ATC cases. The SW1736 cell line was characterized by a high percentage of stem population (10.4±2.1% of cells were aldehyde dehydrogenase positive) and high expression of several CSC markers (SOX2, OCT4, NANOG, C-MYC, and SSEA4). SOX2 silencing down-regulated OCT-4, NANOG, and ABCG2. SOX2 silencing sensitized SW1736 cells, causing a significant cell death increase (1.8-fold) in comparison to control cells with 10 μM cisplatin (93.9±3.4% vs. 52.6±9.4%, p<0.01) and 2.7 fold with 0.5 μM doxorubicin (45.8±9.9% vs. 17.1±3.4% p<0.01). ABCG2 silencing caused increased cell death with both cisplatin (74.9±1.4%) and doxorubicin treatment (74.1±0.1%) vs. no-target-treated cells (respectively, 45.8±1.0% and 48.6±1.0%, p<0.001).
Conclusions: The characterization of CSCs in ATC through the analysis of multiple pluripotent stem cell markers might be useful in identifying cells with a stem-like phenotype capable of resisting conventional chemotherapy. In addition, our data demonstrate that SOX2 switch-off through ABCG2 transporter down-regulation has a major role in overcoming CSC chemotherapy resistance.
Figures
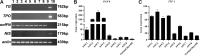


References
-
- Gharib H. Papini E. Paschke R. Thyroid nodules: a review of current guidelines, practices, and prospects. Eur J Endocrinol. 2008;159:493–505. - PubMed
-
- Thomas T. Nowka K. Lan L. Derwahl M. Expression of endoderm stem cell markers: evidence for the presence of adult stem cells in human thyroid. Thyroid. 2006;16:537–544. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

