Integral membrane proteins and bilayer proteomics
- PMID: 23301778
- PMCID: PMC3664232
- DOI: 10.1021/ac303064a
Integral membrane proteins and bilayer proteomics
Abstract
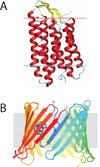
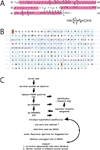
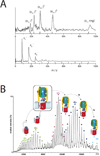
Integral membrane proteins reside within the bilayer membranes that surround cells and organelles, playing critical roles in movement of molecules across them and the transduction of energy and signals. While their extreme amphipathicity presents technical challenges, biological mass spectrometry has been applied to all aspects of membrane protein chemistry and biology, including analysis of primary, secondary, tertiary, and quaternary structures as well as the dynamics that accompany functional cycles and catalysis.
Figures


References
-
- Fagerberg L, Jonasson K, von Heijne G, Uhlén M, Berglund L. Proteomics. 2010;10(6):1141–1149. - PubMed
-
- Weinglass AB, Whitelegge JP, Kaback HR. Curr. Opin. Drug Discov. Devel. 2004;7(5):589–599. - PubMed
-
- von Heijne G. Annu. Rev. Biochem. 2011;80:157–160. - PubMed
-
- von Heijne G. Biochem. Soc. Trans. 2011;39(3):747–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

