Bioactive flavaglines and other constituents isolated from Aglaia perviridis
- PMID: 23301897
- PMCID: PMC3606667
- DOI: 10.1021/np3007588
Bioactive flavaglines and other constituents isolated from Aglaia perviridis
Abstract
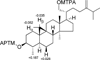
Eight new compounds, including two cyclopenta[b]benzopyran derivatives (1, 2), two cyclopenta[b]benzofuran derivatives (3, 4), three cycloartane triterpenoids (5-7), and an apocarotenoid (8), together with 16 known compounds, were isolated from the chloroform-soluble partitions of separate methanol extracts of a combination of the fruits, leaves, and twigs and of the roots of Aglaia perviridis collected in Vietnam. Isolation work was monitored using human colon cancer cells (HT-29) and facilitated with an LC/MS dereplication procedure. The structures of the new compounds (1-8) were determined on the basis of spectroscopic data interpretation. The Mosher ester method was employed to determine the absolute configurations of 5-7, and the absolute configuration of the 9,10-diol unit of compound 8 was established by a dimolybdenum tetraacetate [Mo2(AcO)4] induced circular dichroism procedure. Seven known rocaglate derivatives (9-15) exhibited significant cytotoxicity against the HT-29 cell line, with rocaglaol (9) being the most potent (ED50 0.0007 μM). The new compounds 2-4 were also active against this cell line, with ED50 values ranging from 0.46 to 4.7 μM. The cytotoxic compounds were evaluated against a normal colon cell line, CCD-112CoN. In addition, the new compound perviridicin B (2), three known rocaglate derivatives (9, 11, 12), and a known sesquiterpene, 2-oxaisodauc-5-en-12-al (17), showed significant NF-κB (p65) inhibitory activity in an ELISA assay.
Figures

 ) and NOESY (
) and NOESY ( ) correlations observed for perviridisin A (1)
) correlations observed for perviridisin A (1)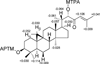


 ) ECD spectrum of compound 8 in a DMSO solution. (
) ECD spectrum of compound 8 in a DMSO solution. ( ) ECD spectrum of compound 8 in a DMSO solution of Mo2(OAc)4 (the inherent ECD was subtracted).
) ECD spectrum of compound 8 in a DMSO solution of Mo2(OAc)4 (the inherent ECD was subtracted).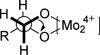
References
-
- Pannell CM. A Taxonomic Monograph of the Genus Aglaia Lour (Meliaceae) Kew, Richmond, Surrey, UK: Kew Bulletin Additional Series XVI; HMSO; 1992.
-
- Proksch P, Edrada RA, Ebel R, Bohnenstengel FI, Nugroho BW. Curr. Org. Chem. 2001;5:923–938.
-
- Kim S, Salim A, Swanson SM, Kinghorn AD. Anti-Cancer Agents Med. Chem. 2006;6:319–345. - PubMed
-
- Ribeiro N, Thuaud F, Nebigil C, Désaubry L. Bioorg. Med. Chem. 2012;20:1857–1864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

