Population-based outcomes after brain radiotherapy in patients with brain metastases from breast cancer in the Pre-Trastuzumab and Trastuzumab eras
- PMID: 23302543
- PMCID: PMC3582534
- DOI: 10.1186/1748-717X-8-12
Population-based outcomes after brain radiotherapy in patients with brain metastases from breast cancer in the Pre-Trastuzumab and Trastuzumab eras
Abstract
Purpose: To evaluate the survival of patients with human epidermal growth factor receptor 2 (HER2) positive and negative metastatic breast cancer irradiated for brain metastases before and after the availability of trastuzumab (T).
Materials and methods: Women diagnosed with brain metastasis from breast cancer in two eras between 2000 and 2007 (T-era, n = 441) and 1986 to 1992 (PreT-era, n = 307), treated with whole brain radiotherapy (RT) were identified. In the T-era, HER2 testing was part of routine clinical practice, and in the preT-era 128/307 (42%) cases had HER2 testing performed retrospectively on tissue microarrays. Overall survival (OS) was estimated using the Kaplan-Meier method and comparisons between eras used log-rank tests.
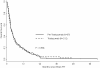
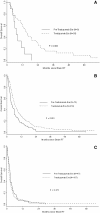
Results: In the preT- and T-era cohorts, the rate of HER2 positivity was 40% (176/441) and 26% (33/128) (p < 0.001). The median time from diagnosis to brain RT was longer in the preT-era (3.3 years versus 2.3 years, p < 0.001). Survival after brain RT was improved in the T-era compared to the preT-era (1-year OS 26% versus 12%, p < 0.001). The 1-year OS rate for HER2 negative patients was 20% in both eras (p = 0.97). Among HER2 positive patients, the 1-year OS in the preT-era was 5% compared to 40% in the T-era (p < 0.001).
Conclusions: Distinct from patients with HER2 negative disease in whom no difference in survival after brain RT was observed over time, patients with HER2 positive brain metastases experienced significantly improved survival subsequent to the availability of trastuzumab.
Figures
References
-
- Chia SK, Speers CH, D’yachkova Y, Kang A, Malfair-Taylor S, Barnett J, Coldman A, Gelmon KA, O’reilly SE, Olivotto IA. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. doi: 10.1002/cncr.22867. - DOI - PubMed
-
- Tsao MN, Lloyd NS, Wong RK, Rakovitch E, Chow E, Laperriere N. Supportive Care Guidelines Group of Cancer Care Ontario’s Program in Evidence-based Care. Radiotherapeutic management of brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2005;31:256–273. doi: 10.1016/j.ctrv.2005.04.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous