Cardiomyocyte proliferation contributes to heart growth in young humans
- PMID: 23302686
- PMCID: PMC3557060
- DOI: 10.1073/pnas.1214608110
Cardiomyocyte proliferation contributes to heart growth in young humans
Abstract
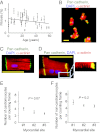
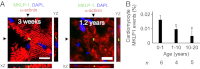
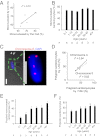
The human heart is believed to grow by enlargement but not proliferation of cardiomyocytes (heart muscle cells) during postnatal development. However, recent studies have shown that cardiomyocyte proliferation is a mechanism of cardiac growth and regeneration in animals. Combined with evidence for cardiomyocyte turnover in adult humans, this suggests that cardiomyocyte proliferation may play an unrecognized role during the period of developmental heart growth between birth and adolescence. We tested this hypothesis by examining the cellular growth mechanisms of the left ventricle on a set of healthy hearts from humans aged 0-59 y (n = 36). The percentages of cardiomyocytes in mitosis and cytokinesis were highest in infants, decreasing to low levels by 20 y. Although cardiomyocyte mitosis was detectable throughout life, cardiomyocyte cytokinesis was not evident after 20 y. Between the first year and 20 y of life, the number of cardiomyocytes in the left ventricle increased 3.4-fold, which was consistent with our predictions based on measured cardiomyocyte cell cycle activity. Our findings show that cardiomyocyte proliferation contributes to developmental heart growth in young humans. This suggests that children and adolescents may be able to regenerate myocardium, that abnormal cardiomyocyte proliferation may be involved in myocardial diseases that affect this population, and that these diseases might be treatable through stimulation of cardiomyocyte proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

