LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1
- PMID: 23302695
- PMCID: PMC3557093
- DOI: 10.1073/pnas.1211179110
LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1
Erratum in
-
Correction for Ren et al., LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):E7643-E7644. doi: 10.1073/pnas.1616960113. Epub 2016 Nov 14. Proc Natl Acad Sci U S A. 2016. PMID: 27849597 Free PMC article. No abstract available.
Abstract
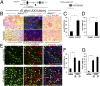
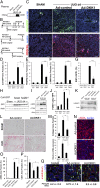
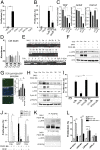
Fibrosis of vital organs is a major public health problem with limited therapeutic options. Mesenchymal cells including microvascular mural cells (pericytes) are major progenitors of scar-forming myofibroblasts in kidney and other organs. Here we show pericytes in healthy kidneys have active WNT/β-catenin signaling responses that are markedly up-regulated following kidney injury. Dickkopf-related protein 1 (DKK-1), a ligand for the WNT coreceptors low-density lipoprotein receptor-related proteins 5 and 6 (LRP-5 and LRP-6) and an inhibitor of WNT/β-catenin signaling, effectively inhibits pericyte activation, detachment, and transition to myofibroblasts in vivo in response to kidney injury, resulting in attenuated fibrogenesis, capillary rarefaction, and inflammation. DKK-1 blocks activation and proliferation of established myofibroblasts in vitro and blocks pericyte proliferation to PDGF, pericyte migration, gene activation, and cytoskeletal reorganization to TGF-β or connective tissue growth factor. These effects are largely independent of inhibition of downstream β-catenin signaling. DKK-1 acts predominantly by inhibiting PDGF-, TGF-β-, and connective tissue growth factor-activated MAPK and JNK signaling cascades, acting via LRP-6 with associated WNT ligand. Biochemically, LRP-6 interacts closely with PDGF receptor β and TGF-β receptor 1 at the cell membrane, suggesting that it may have roles in pathways other than WNT/β-catenin. In summary, DKK-1 blocks many of the changes in pericytes required for myofibroblast transition and attenuates established myofibroblast proliferation/activation by mechanisms dependent on LRP-6 and WNT ligands but not the downstream β-catenin pathway.
Conflict of interest statement
Conflict of interest statement: S.R. and J.S.D have submitted patent applications for the use of DKK-1 in fibrosis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

