Comparative effects of vaccination against porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) in a PCV2-PRRSV challenge model
- PMID: 23302743
- PMCID: PMC3592349
- DOI: 10.1128/CVI.00497-12
Comparative effects of vaccination against porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) in a PCV2-PRRSV challenge model
Abstract
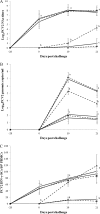
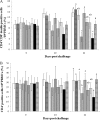
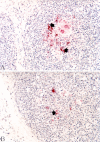
The objective of the present study was to determine the effects of porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) vaccinations in an experimental PCV2-PRRSV challenge model, based on virological (viremia), immunological (neutralizing antibodies [NAs], gamma interferon-secreting cells [IFN-γ-SCs], and CD4(+) CD8(+) double-positive cells), and pathological (lesions and antigens in lymph nodes and lungs) evaluations. A total of 72 pigs were randomly divided into 9 groups (8 pigs per group): 5 vaccinated and challenged groups, 3 nonvaccinated and challenged groups, and a negative-control group. Vaccination against PCV2 induced immunological responses (NAs and PCV2-specific IFN-γ-SCs) and reduced PCV2 viremia, PCV2-induced lesions, and PCV2 antigens in the dually infected pigs. However, vaccination against PCV2 did not affect the PRRSV immunological responses (NAs and PRRSV-specific IFN-γ-SCs), PRRSV viremia, PRRSV-induced lesions, or PRRSV antigens in the dually infected pigs. Vaccination against PRRSV did not induce immunological responses (PRRSV-specific IFN-γ-SCs) or reduce PRRSV viremia, PRRSV-induced lesions, or PRRSV antigens in the dually infected pigs. In addition, vaccination against PRRSV increased PCV2 viremia, PCV2-induced lesions, and PCV2 antigens in the dually infected pigs. In summary, vaccination against PCV2 reduced PCV2 viremia, PCV2-induced lesions, and PCV2 antigens in the dually infected pigs. However, vaccination against PRRSV increased PCV2 viremia, PCV2-induced lesions, and PCV2 antigens in the dually infected pigs. Therefore, the PCV2 vaccine decreased the potentiation of PCV2-induced lesions by PRRSV in dually infected pigs. In contrast, the PRRSV vaccine alone did not decrease the potentiation of PCV2-induced lesions by PRRSV in dually infected pigs.
Figures


Similar articles
-
Comparison of four commercial one-dose porcine circovirus type 2 (PCV2) vaccines administered to pigs challenged with PCV2 and porcine reproductive and respiratory syndrome virus at 17 weeks postvaccination to control porcine respiratory disease complex under Korean field conditions.Clin Vaccine Immunol. 2014 Mar;21(3):399-406. doi: 10.1128/CVI.00768-13. Epub 2014 Jan 8. Clin Vaccine Immunol. 2014. PMID: 24403524 Free PMC article.
-
Concurrent vaccinations against PCV2 and PRRSV: study on the specific immunity and clinical protection in naturally infected pigs.Vet Microbiol. 2013 Mar 23;162(2-4):558-571. doi: 10.1016/j.vetmic.2012.11.016. Epub 2012 Nov 14. Vet Microbiol. 2013. PMID: 23206413 Clinical Trial.
-
Porcine reproductive and respiratory syndrome virus infection at the time of porcine circovirus type 2 vaccination has no impact on vaccine efficacy.Clin Vaccine Immunol. 2010 Dec;17(12):1940-5. doi: 10.1128/CVI.00338-10. Epub 2010 Oct 6. Clin Vaccine Immunol. 2010. PMID: 20926694 Free PMC article.
-
Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae.Vet J. 2016 Jun;212:1-6. doi: 10.1016/j.tvjl.2015.10.030. Epub 2015 Oct 23. Vet J. 2016. PMID: 27256017 Review.
-
[The porcine respiratory disease complex (PRDC) - a clinical review].Tierarztl Prax Ausg G Grosstiere Nutztiere. 2021 Apr;49(2):120-132. doi: 10.1055/a-1403-1976. Epub 2021 Apr 26. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2021. PMID: 33902142 Review. German.
Cited by
-
Protein composition of bronchoalveolar lavage fluid and airway surface liquid from newborn pigs.Am J Physiol Lung Cell Mol Physiol. 2013 Aug 1;305(3):L256-66. doi: 10.1152/ajplung.00056.2013. Epub 2013 May 24. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23709621 Free PMC article.
-
Comparison of four commercial one-dose porcine circovirus type 2 (PCV2) vaccines administered to pigs challenged with PCV2 and porcine reproductive and respiratory syndrome virus at 17 weeks postvaccination to control porcine respiratory disease complex under Korean field conditions.Clin Vaccine Immunol. 2014 Mar;21(3):399-406. doi: 10.1128/CVI.00768-13. Epub 2014 Jan 8. Clin Vaccine Immunol. 2014. PMID: 24403524 Free PMC article.
-
A field efficacy trial of a trivalent vaccine containing porcine circovirus type 2a and 2b, and Mycoplasma hyopneumoniae in three herds.Vet Med Sci. 2022 Mar;8(2):578-590. doi: 10.1002/vms3.657. Epub 2021 Oct 22. Vet Med Sci. 2022. PMID: 34687172 Free PMC article.
-
Porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus alone or associated are frequent intralesional detected viruses in porcine respiratory disease complex cases in Northern Italy.Front Vet Sci. 2023 Aug 31;10:1234779. doi: 10.3389/fvets.2023.1234779. eCollection 2023. Front Vet Sci. 2023. PMID: 37720469 Free PMC article.
-
Experimental reproduction of porcine respiratory disease complex in pigs inoculated porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae and followed by inoculation with porcine circovirus type 2.J Vet Med Sci. 2021 Mar 11;83(3):427-430. doi: 10.1292/jvms.20-0577. Epub 2021 Jan 19. J Vet Med Sci. 2021. PMID: 33473060 Free PMC article.
References
-
- Chae C. 2004. Postweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathology. Vet. J. 168:41–49 - PubMed
-
- Chae C. 2005. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 169:326–336 - PubMed
-
- Cavanagh D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 142:629–633 - PubMed
-
- Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorell M. 2012. Porcine reproductive and respiratory syndrome virus (porcine arterivirus), p 461–486. In Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW. (ed), Diseases of swine, 10th ed Wiley-Blackwell, West Sussex, United Kingdom
-
- Kim J, Chung HK, Chae C. 2003. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet. J. 166:251–256 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

