Properties of two cataract-associated mutations located in the NH2 terminus of connexin 46
- PMID: 23302783
- PMCID: PMC3651606
- DOI: 10.1152/ajpcell.00344.2012
Properties of two cataract-associated mutations located in the NH2 terminus of connexin 46
Abstract
Mutations in connexin 46 are associated with congenital cataracts. The purpose of this project was to characterize cellular and functional properties of two congenital cataract-associated mutations located in the NH2 terminus of connexin 46: Cx46D3Y and Cx46L11S, which we found localized to gap junctional plaques like wild-type Cx46 in transfected HeLa cells. Dual two-microelectrode-voltage-clamp studies of Xenopus oocyte pairs injected with wild-type or mutant rat Cx46 showed that oocyte pairs injected with D3Y or L11S cRNA failed to induce gap junctional coupling, whereas oocyte pairs injected with Cx46 showed high levels of coupling. D3Y, but not L11S, functionally paired with wild-type Cx46. To determine whether coexpression of D3Y or L11S affected the junctional conductance produced by wild-type lens connexins, we studied pairs of oocytes coinjected with equal amounts of mutant and wild-type connexin cRNA. Expression of D3Y or L11S almost completely abolished gap junctional coupling induced by Cx46. In contrast, expression of D3Y or L11S failed to inhibit junctional conductance induced by Cx50. To examine effects of the D3Y and L11S mutations on hemichannel activity, hemichannel currents were measured in connexin cRNA-injected oocytes. Oocytes expressing D3Y exhibited reduced hemichannel activity as well as alterations in voltage gating and charge selectivity while oocytes expressing L11S showed no hemichannel activity. Moreover, coexpression of mutant with wild-type Cx50 or Cx46 gave rise to hemichannels with distinct electrophysiological properties, suggesting that the mutant connexins were forming heteromeric channels with wild-type connexins. These data suggest D3Y and L11S cause cataracts by similar but not identical mechanisms.
Keywords: cataract; connexin; gap junction; intercellular communication.
Figures
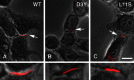
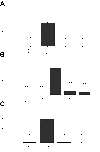






Comment in
-
Is half of a lens gap junction channel better than none? Focus on "properties of two cataract-associated mutations located in the NH2 terminus of connexin 46".Am J Physiol Cell Physiol. 2013 May 1;304(9):C821-2. doi: 10.1152/ajpcell.00035.2013. Epub 2013 Feb 7. Am J Physiol Cell Physiol. 2013. PMID: 23392114 No abstract available.
References
-
- Addison PK, Berry V, Holden KR, Espinal D, Rivera B, Su H, Srivastava AK, Bhattacharya SS. A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol Vis 12: 791–795, 2006 - PubMed
-
- Arora A, Minogue PJ, Liu X, Reddy MA, Ainsworth JR, Bhattacharya SS, Webster AR, Hunt DM, Ebihara L, Moore AT, Beyer EC, Berthoud VM. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet 43: e2, 2006 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

