CD22 is required for protection against West Nile virus Infection
- PMID: 23302871
- PMCID: PMC3592166
- DOI: 10.1128/JVI.02368-12
CD22 is required for protection against West Nile virus Infection
Abstract
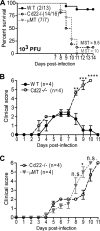
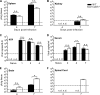
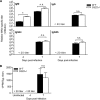
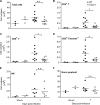
West Nile virus (WNV) is a RNA virus of the family Flaviviridae and the leading cause of mosquito-borne encephalitis in the United States. Humoral immunity is essential for protection against WNV infection; however, the requirements for initiating effective antibody responses against WNV infection are still unclear. CD22 (Siglec-2) is expressed on B cells and regulates B cell receptor signaling, cell survival, proliferation, and antibody production. In this study, we investigated how CD22 contributes to protection against WNV infection and found that CD22 knockout (Cd22(-/-)) mice were highly susceptible to WNV infection and had increased viral loads in the serum and central nervous system (CNS) compared to wild-type (WT) mice. This was not due to a defect in humoral immunity, as Cd22(-/-) mice had normal WNV-specific antibody responses. However, Cd22(-/-) mice had decreased WNV-specific CD8(+) T cell responses compared to those of WT mice. These defects were not simply due to reduced cytotoxic activity or increased cell death but, rather, were associated with decreased lymphocyte migration into the draining lymph nodes (dLNs) of infected Cd22(-/-) mice. Cd22(-/-) mice had reduced production of the chemokine CCL3 in the dLNs after infection, suggesting that CD22 affects chemotaxis via controlling chemokine production. CD22 was not restricted to B cells but was also expressed on a subset of splenic DCIR2(+) dendritic cells that rapidly expand early after WNV infection. Thus, CD22 plays an essential role in controlling WNV infection by governing cell migration and CD8(+) T cell responses.
Figures




Similar articles
-
Dynamics of Tissue-Specific CD8+ T Cell Responses during West Nile Virus Infection.J Virol. 2018 Apr 27;92(10):e00014-18. doi: 10.1128/JVI.00014-18. Print 2018 May 15. J Virol. 2018. PMID: 29514902 Free PMC article.
-
Interferon regulatory factor 5-dependent immune responses in the draining lymph node protect against West Nile virus infection.J Virol. 2014 Oct;88(19):11007-21. doi: 10.1128/JVI.01545-14. Epub 2014 Jul 16. J Virol. 2014. PMID: 25031348 Free PMC article.
-
Tumor necrosis factor alpha protects against lethal West Nile virus infection by promoting trafficking of mononuclear leukocytes into the central nervous system.J Virol. 2008 Sep;82(18):8956-64. doi: 10.1128/JVI.01118-08. Epub 2008 Jul 16. J Virol. 2008. PMID: 18632856 Free PMC article.
-
The host immunologic response to West Nile encephalitis virus.Front Biosci (Landmark Ed). 2009 Jan 1;14(8):3024-34. doi: 10.2741/3432. Front Biosci (Landmark Ed). 2009. PMID: 19273254 Review.
-
B cell response and mechanisms of antibody protection to West Nile virus.Viruses. 2014 Mar 3;6(3):1015-36. doi: 10.3390/v6031015. Viruses. 2014. PMID: 24594676 Free PMC article. Review.
Cited by
-
Novel approaches and challenges to treatment of central nervous system viral infections.Ann Neurol. 2013 Sep;74(3):412-22. doi: 10.1002/ana.23988. Ann Neurol. 2013. PMID: 23913580 Free PMC article. Review.
-
CD22: A Regulator of Innate and Adaptive B Cell Responses and Autoimmunity.Front Immunol. 2018 Sep 28;9:2235. doi: 10.3389/fimmu.2018.02235. eCollection 2018. Front Immunol. 2018. PMID: 30323814 Free PMC article. Review.
-
Regulatory T cells shape the resident memory T cell response to virus infection in the tissues.J Immunol. 2014 Jan 15;192(2):683-90. doi: 10.4049/jimmunol.1202153. Epub 2013 Dec 11. J Immunol. 2014. PMID: 24337378 Free PMC article.
-
Sialic Acids in the Immune Response during Sepsis.Front Immunol. 2017 Nov 21;8:1601. doi: 10.3389/fimmu.2017.01601. eCollection 2017. Front Immunol. 2017. PMID: 29209331 Free PMC article. Review.
-
Single-cell analysis reveals an antiviral network that controls Zika virus infection in human dendritic cells.J Virol. 2024 May 14;98(5):e0019424. doi: 10.1128/jvi.00194-24. Epub 2024 Apr 3. J Virol. 2024. PMID: 38567950 Free PMC article.
References
-
- Dörken B, Moldenhauer G, Pezzutto A, Schwartz R, Feller A, Kiesel S, Nadler LM. 1986. HD39 (B3), a B lineage-restricted antigen whose cell surface expression is limited to resting and activated human B lymphocytes. J. Immunol. 136:4470–4479 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

