Adenovirus L-E1A activates transcription through mediator complex-dependent recruitment of the super elongation complex
- PMID: 23302885
- PMCID: PMC3592126
- DOI: 10.1128/JVI.03046-12
Adenovirus L-E1A activates transcription through mediator complex-dependent recruitment of the super elongation complex
Abstract
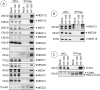
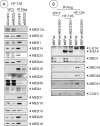
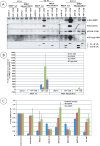
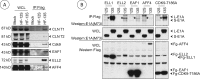
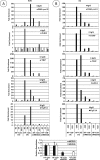
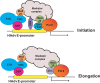
The adenovirus large E1A (L-E1A) protein is a prototypical transcriptional activator, and it functions through the action of a conserved transcriptional activation domain, CR3. CR3 interacts with a mediator subunit, MED23, that has been linked to the transcriptional activity of CR3. Our unbiased proteomic analysis revealed that human adenovirus 5 (HAdv5) L-E1A was associated with many mediator subunits. In MED23-depleted cells and in Med23 knockout (KO) cells, L-E1A was deficient in association with other mediator subunits, suggesting that MED23 links CR3 with the mediator complex. Short interfering RNA (siRNA)-mediated depletion of several mediator subunits suggested differential effects of various subunits on transcriptional activation of HAdv5 early genes. In addition to MED23, mediator subunits such as MED14 and MED26 were also essential for the transcription of HAdv5 early genes. The L-E1A proteome contained MED26-associated super elongation complex. The catalytic component of the elongation complex, CDK9, was important for the transcriptional activity of L-E1A and HAdv5 replication. Our results suggest that L-E1A-mediated transcriptional activation involves a transcriptional elongation step, like HIV Tat, and constitutes a therapeutic target for inhibition of HAdv replication.
Figures

References
-
- Berk AJ. 2005. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 24:7673–7685 - PubMed
-
- Berk AJ, Lee F, Harrison T, Williams J, Sharp PA. 1979. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell 17:935–944 - PubMed
-
- Green M, Loewenstein PM, Pusztai R, Symington JS. 1988. An adenovirus E1A protein domain activates transcription in vivo and in vitro in the absence of protein synthesis. Cell 53:921–926 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

