Role of interactions between Autographa californica multiple nucleopolyhedrovirus procathepsin and chitinase chitin-binding or active-site domains in viral cathepsin processing
- PMID: 23302896
- PMCID: PMC3592168
- DOI: 10.1128/JVI.01937-12
Role of interactions between Autographa californica multiple nucleopolyhedrovirus procathepsin and chitinase chitin-binding or active-site domains in viral cathepsin processing
Abstract
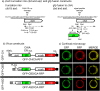
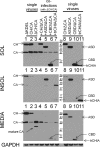
The binding of Autographa californica multiple nucleopolyhedrovirus chitinase (CHIA) to viral cathepsin protease progenitor (proV-CATH) governs cellular/endoplasmic reticulum (ER) coretention of CHIA and proV-CATH, thus coordinating simultaneous cellular release of both host tissue-degrading enzymes upon host cell death. CHIA is a proposed proV-CATH folding chaperone because insertional inactivation of chiA causes production of proV-CATH aggregates that are incompetent for proteolytic maturation into active V-CATH enzyme. We wanted to determine whether the N-terminal chitin-binding domain (CBD, 149 residues) and C-terminal CHIA active-site domain (ASD, 402 residues) of CHIA bind to proV-CATH independently of one another and whether either domain is dispensable for CHIA's putative proV-CATH folding chaperone activity. We demonstrate that N-terminally green fluorescent protein (GFP)-fused CHIA, ASD, and CBD each colocalize with proV-CATH-RFP in ER-like patterns and that both ASD and CBD independently associate with proV-CATH in vivo using bimolecular fluorescence complementation (BiFC) and in vitro using reciprocal nickel-histidine pulldown assays. Altogether, the data from colocalization, BiFC, and reciprocal copurification analyses suggest specific and independent interactions between proV-CATH and both domains of CHIA. These data also demonstrate that either CHIA domain is dispensable for normal proV-CATH processing. Furthermore, in contrast to prior evidence suggesting that a lack of chiA expression causes proV-CATH to become aggregated, insoluble, and unable to mature into V-CATH, a chiA deletion bacmid virus we engineered to express just v-cath produced soluble proV-CATH that was prematurely secreted from cells and proteolytically matured into active V-CATH enzyme.
Figures






References
-
- Hom LG, Ohkawa T, Trudeau D, Volkman LE. 2002. Autographa californica M nucleopolyhedrovirus ProV-CATH is activated during infected cell death. Virology 296:212–218 - PubMed
-
- Hawtin RE, Zarkowska T, Arnold K, Thomas CJ, Gooday GW, King LA, Kuzio JA, Possee RD. 1997. Liquefaction of Autographa californica nucleopolyhedrovirus-infected insects is dependent on the integrity of virus-encoded chitinase and cathepsin genes. Virology 238:243–253 - PubMed
-
- Wang F, Zhang CX, Shyam Kumar V, Wu XF. 2005. Influences of chitinase gene deletion from BmNPV on the cell lysis and host liquefaction. Arch. Virol. 150:981–990 - PubMed
-
- Hom LG, Volkman LE. 2000. Autographa californica M nucleopolyhedrovirus chiA is required for processing of V-CATH. Virology 277:178–183 - PubMed
-
- Daimon T, Katsuma S, Shimada T. 2007. Mutational analysis of active site residues of chitinase from Bombyx mori nucleopolyhedrovirus. Virus Res. 124:168–175 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

