Enhanced histaminergic neurotransmission and sleep-wake alterations, a study in histamine H3-receptor knock-out mice
- PMID: 23303066
- PMCID: PMC3629391
- DOI: 10.1038/npp.2012.266
Enhanced histaminergic neurotransmission and sleep-wake alterations, a study in histamine H3-receptor knock-out mice
Abstract
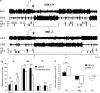
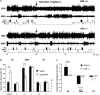
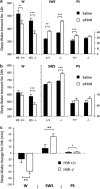
Long-term abolition of a brain arousal system impairs wakefulness (W), but little is known about the consequences of long-term enhancement. The brain histaminergic arousal system is under the negative control of H3-autoreceptors whose deletion results in permanent enhancement of histamine (HA) turnover. In order to determine the consequences of enhancement of the histaminergic system, we compared the cortical EEG and sleep-wake states of H3-receptor knockout (H3R-/-) and wild-type mouse littermates. We found that H3R-/-mice had rich phenotypes. On the one hand, they showed clear signs of enhanced HA neurotransmission and vigilance, i.e., a higher EEG θ power during spontaneous W and a greater extent of W or sleep restriction during behavioral tasks, including environmental change, locomotion, and motivation tests. On the other hand, during the baseline dark period, they displayed deficient W and signs of sleep deterioration, such as pronounced sleep fragmentation and reduced cortical slow activity during slow wave sleep (SWS), most likely due to a desensitization of postsynaptic histaminergic receptors as a result of constant HA release. Ciproxifan (H3-receptor inverse agonist) enhanced W in wild-type mice, but not in H3R-/-mice, indicating a functional deletion of H3-receptors, whereas triprolidine (postsynaptic H1-receptor antagonist) or α-fluoromethylhistidine (HA-synthesis inhibitor) caused a greater SWS increase in H3R-/- than in wild-type mice, consistent with enhanced HA neurotransmission. These sleep-wake characteristics and the obesity phenotypes previously reported in this animal model suggest that chronic enhancement of histaminergic neurotransmission eventually compromises the arousal system, leading to sleep-wake, behavioral, and metabolic disorders similar to those caused by voluntary sleep restriction in humans.
Figures






References
-
- Abe H, Honma S, Ohtsu H, Honma K. Circadian rhythms in behavior and clock gene expressions in the brain of mice lacking histidine decarboxylase. Brain Res Mol Brain Res. 2004;124:178–187. - PubMed
-
- Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. - PubMed
-
- Bonaventure P, Letavic M, Dugovic C, Wilson S, Aluisio L, Pudiak C, et al. Histamine H3 receptor antagonists: from target identification to drug leads. Biochem Pharmacol. 2007;73:1084–1096. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

