MDM2, MDMX and p53 in oncogenesis and cancer therapy
- PMID: 23303139
- PMCID: PMC4161369
- DOI: 10.1038/nrc3430
MDM2, MDMX and p53 in oncogenesis and cancer therapy
Abstract
The MDM2 and MDMX (also known as HDMX and MDM4) proteins are deregulated in many human cancers and exert their oncogenic activity predominantly by inhibiting the p53 tumour suppressor. However, the MDM proteins modulate and respond to many other signalling networks in which they are embedded. Recent mechanistic studies and animal models have demonstrated how functional interactions in these networks are crucial for maintaining normal tissue homeostasis, and for determining responses to oncogenic and therapeutic challenges. This Review highlights the progress made and pitfalls encountered as the field continues to search for MDM-targeted antitumour agents.
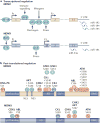
Figures
References
-
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. The first demonstration that MDM2 could inhibit p53 activity. - PubMed
-
- Shibagaki I, et al. p53 mutation, murine double minute 2 amplification, and human papillomavirus infection are frequently involved but not associated with each other in esophageal squamous cell carcinoma. Clin Cancer Res. 1995;1:769–773. - PubMed
-
- Forslund A, et al. MDM2 gene amplification is correlated to tumor progression but not to the presence of SNP309 or TP53 mutational status in primary colorectal cancers. Mol Cancer Res. 2008;6:205–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

