11β-hydroxysteroid dehydrogenase type 1 deficiency in bone marrow-derived cells reduces atherosclerosis
- PMID: 23303209
- PMCID: PMC3606528
- DOI: 10.1096/fj.12-219105
11β-hydroxysteroid dehydrogenase type 1 deficiency in bone marrow-derived cells reduces atherosclerosis
Abstract
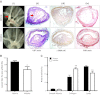
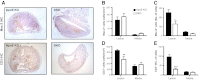
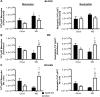
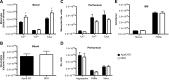
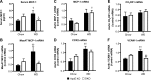
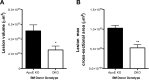
11β-Hydroxysteroid dehydrogenase type-1 (11β-HSD1) converts inert cortisone into active cortisol, amplifying intracellular glucocorticoid action. 11β-HSD1 deficiency improves cardiovascular risk factors in obesity but exacerbates acute inflammation. To determine the effects of 11β-HSD1 deficiency on atherosclerosis and its inflammation, atherosclerosis-prone apolipoprotein E-knockout (ApoE-KO) mice were treated with a selective 11β-HSD1 inhibitor or crossed with 11β-HSD1-KO mice to generate double knockouts (DKOs) and challenged with an atherogenic Western diet. 11β-HSD1 inhibition or deficiency attenuated atherosclerosis (74-76%) without deleterious effects on plaque structure. This occurred without affecting plasma lipids or glucose, suggesting independence from classical metabolic risk factors. KO plaques were not more inflamed and indeed had 36% less T-cell infiltration, associated with 38% reduced circulating monocyte chemoattractant protein-1 (MCP-1) and 36% lower lesional vascular cell adhesion molecule-1 (VCAM-1). Bone marrow (BM) cells are key to the atheroprotection, since transplantation of DKO BM to irradiated ApoE-KO mice reduced atherosclerosis by 51%. 11β-HSD1-null macrophages show 76% enhanced cholesterol ester export. Thus, 11β-HSD1 deficiency reduces atherosclerosis without exaggerated lesional inflammation independent of metabolic risk factors. Selective 11β-HSD1 inhibitors promise novel antiatherosclerosis effects over and above their benefits for metabolic risk factors via effects on BM cells, plausibly macrophages.
Figures

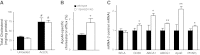
References
-
- Libby P. (2002) Inflammation in atherosclerosis. Nature 420, 868–874 - PubMed
-
- Hansson G. K., Libby P. (2006) The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 6, 508–519 - PubMed
-
- Hansson G. K., Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 - PubMed
-
- Taleb S., Tedgui A., Mallat Z. Adaptive T cell immune responses and atherogenesis. Curr. Opin. Pharmacol. 10, 197–202 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

