Magnetic poly(ε-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering
- PMID: 23303218
- PMCID: PMC3565733
- DOI: 10.1098/rsif.2012.0833
Magnetic poly(ε-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering
Abstract
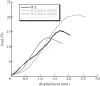
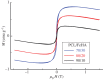
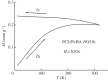
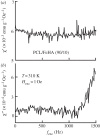
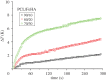
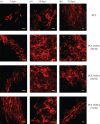
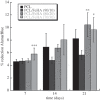
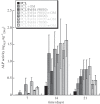
In biomedicine, magnetic nanoparticles provide some attractive possibilities because they possess peculiar physical properties that permit their use in a wide range of applications. The concept of magnetic guidance basically spans from drug delivery and hyperthermia treatment of tumours, to tissue engineering, such as magneto-mechanical stimulation/activation of cell constructs and mechanosensitive ion channels, magnetic cell-seeding procedures, and controlled cell proliferation and differentiation. Accordingly, the aim of this study was to develop fully biodegradable and magnetic nanocomposite substrates for bone tissue engineering by embedding iron-doped hydroxyapatite (FeHA) nanoparticles in a poly(ε-caprolactone) (PCL) matrix. X-ray diffraction analyses enabled the demonstration that the phase composition and crystallinity of the magnetic FeHA were not affected by the process used to develop the nanocomposite substrates. The mechanical characterization performed through small punch tests has evidenced that inclusion of 10 per cent by weight of FeHA would represent an effective reinforcement. The inclusion of nanoparticles also improves the hydrophilicity of the substrates as evidenced by the lower values of water contact angle in comparison with those of neat PCL. The results from magnetic measurements confirmed the superparamagnetic character of the nanocomposite substrates, indicated by a very low coercive field, a saturation magnetization strictly proportional to the FeHA content and a strong history dependence in temperature sweeps. Regarding the biological performances, confocal laser scanning microscopy and AlamarBlue assay have provided qualitative and quantitative information on human mesenchymal stem cell adhesion and viability/proliferation, respectively, whereas the obtained ALP/DNA values have shown the ability of the nanocomposite substrates to support osteogenic differentiation.
Figures






References
-
- Langer R, Vacanti JP. 1993. Tissue engineering. Science 260, 920–92610.1126/science.8493529 (doi:10.1126/science.8493529) - DOI - DOI - PubMed
-
- Chapekar MS. 2000. Tissue engineering: challenges and opportunities. J. Biomed. Mater. Res. B Appl. Biomater. 53, 617–62010.1002/1097-4636(2000)53:6<617::AID-JBM1>3.0.CO;2-C (doi:10.1002/1097-4636(2000)53:6<617::AID-JBM1>3.0.CO;2-C) - DOI - DOI - PubMed
-
- Cheung HY, Lau KT, Lu TP, Hui D. 2007. A critical review on polymer-based bioengineered materials for scaffold development. Compos. B Eng. 38, 291–30010.1016/j.compositesb.2006.06.014 (doi:10.1016/j.compositesb.2006.06.014) - DOI - DOI
-
- Gloria A, De Santis R, Ambrosio L. 2010. Polymer-based composite scaffolds for tissue engineering. J. Appl. Biomater. Biomech. 8, 57–67 - PubMed
-
- Gloria A, Russo T, De Santis R, Ambrosio L. 2009. 3D fiber deposition technique to make multifunctional and tailor-made scaffolds for tissue engineering applications. J. Appl. Biomater. Biomech. 7, 141–152 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

