Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates
- PMID: 23303281
- PMCID: PMC3618620
- DOI: 10.1038/gt.2012.101
Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates
Erratum in
- Gene Ther. 2013 Apr;20(4):465
Abstract
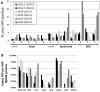
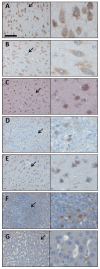
Injection of adeno-associated virus (AAV) into the cerebrospinal fluid (CSF) offers a means to achieve widespread transgene delivery to the central nervous system, where the doses can be readily translated from small to large animals. In contrast to studies with other serotypes (AAV2, AAV4 and AAV5) in rodents, we report that a naturally occurring capsid (AAV9) and rationally engineered capsid (AAV2.5) are able to achieve broad transduction throughout the brain and spinal cord parenchyma following a single injection into the CSF (via cisterna magna or lumbar cistern) in non-human primates (NHP). Using either vector at a dose of ∼2 × 10(12) vector genome (vg) per 3-6 kg animal, approximately 2% of the entire brain and spinal cord was transduced, covering all regions of the central nervous system (CNS). AAV9 in particular displayed efficient transduction of spinal cord motor neurons. The peripheral organ biodistribution was highly reduced compared with intravascular delivery, and the presence of circulating anti-AAV-neutralizing antibodies up to a 1:128 titer had no inhibitory effect on CNS gene transfer. Intra-CSF delivery effectively translates from rodents to NHPs, which provides encouragement for the use of this approach in humans to treat motor neuron and lysosomal storage diseases.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

