Regulation of neurite growth by tumour necrosis superfamily member RANKL
- PMID: 23303310
- PMCID: PMC3603457
- DOI: 10.1098/rsob.120150
Regulation of neurite growth by tumour necrosis superfamily member RANKL
Abstract
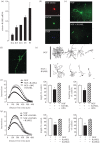
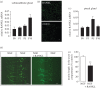
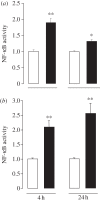
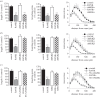
RANKL (receptor-activator of NF-κB ligand, TNFSF11) is a member of the TNF superfamily that regulates bone remodelling and the development of the thymus, lymph nodes and mammary glands. While RANKL and its membrane bound receptor RANK (TNFRSF11A) are expressed in the adult central nervous system and have been implicated in thermoregulation, the potential function of RANK signalling in the developing nervous system remains unexplored. Here, we show that RANK is expressed by sympathetic and sensory neurons of the developing mouse peripheral nervous system and that activating RANK signalling in these neurons during perinatal development by either treating cultured neurons with soluble RANKL or overexpressing RANK in the neurons inhibited neurotrophin-promoted neurite growth without affecting neurotrophin-promoted neuronal survival. RANKL is expressed in tissues innervated by these neurons, and studies in compartment cultures demonstrated that RANKL is capable of acting directly on neurites to inhibit growth locally. Enhancing RANK signalling in cultured neurons resulted in NF-κB activation and phosphorylation of the p65 NF-κB subunit on serine 536. Transfecting neurons with a series of mutated signalling proteins showed that NF-κB activation and p65 phosphorylation occurred by an IKKβ-dependent mechanism and that blockade of this signalling pathway prevented neurite growth inhibition by RANKL. These findings reveal that RANKL is a novel negative regulator of neurite growth from developing PNS neurons and that it exerts its effects by IKKβ-dependent activation of NF-κB.
Figures


References
-
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. 2003. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 26, 509–56310.1146/annurev.neuro.26.010302.081139 (doi:10.1146/annurev.neuro.26.010302.081139) - DOI - DOI - PubMed
-
- Glebova NO, Ginty DD. 2005. Growth and survival signals controlling sympathetic nervous system development. Annu. Rev. Neurosci. 28, 191–22210.1146/annurev.neuro.28.061604.135659 (doi:10.1146/annurev.neuro.28.061604.135659) - DOI - DOI - PubMed
-
- Davies AM. 2009. Extracellular signals regulating sympathetic neuron survival and target innervation during development. Auton. Neurosci. 151, 39–4510.1016/j.autneu.2009.07.011 (doi:10.1016/j.autneu.2009.07.011) - DOI - DOI - PubMed
-
- Glebova NO, Ginty DD. 2004. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J. Neurosci. 24, 743–75110.1523/JNEUROSCI.4523-03.2004 (doi:10.1523/JNEUROSCI.4523-03.2004) - DOI - DOI - PMC - PubMed
-
- Davies AM. 2003. Regulation of neuronal survival and death by extracellular signals during development. Embo j. 22, 2537–254510.1093/emboj/cdg254 (doi:10.1093/emboj/cdg254) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
