A modified HSP70 inhibitor shows broad activity as an anticancer agent
- PMID: 23303345
- PMCID: PMC3606282
- DOI: 10.1158/1541-7786.MCR-12-0547-T
A modified HSP70 inhibitor shows broad activity as an anticancer agent
Abstract
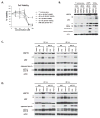
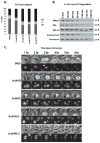
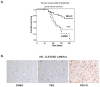
The stress-induced HSP70 is an ATP-dependent molecular chaperone that plays a key role in refolding misfolded proteins and promoting cell survival following stress. HSP70 is marginally expressed in nontransformed cells, but is greatly overexpressed in tumor cells. Silencing HSP70 is uniformly cytotoxic to tumor but not normal cells; therefore, there has been great interest in the development of HSP70 inhibitors for cancer therapy. Here, we report that the HSP70 inhibitor 2-phenylethynesulfonamide (PES) binds to the substrate-binding domain of HSP70 and requires the C-terminal helical "lid" of this protein (amino acids 573-616) to bind. Using molecular modeling and in silico docking, we have identified a candidate binding site for PES in this region of HSP70, and we identify point mutants that fail to interact with PES. A preliminary structure-activity relationship analysis has revealed a derivative of PES, 2-(3-chlorophenyl) ethynesulfonamide (PES-Cl), which shows increased cytotoxicity and ability to inhibit autophagy, along with significantly improved ability to extend the life of mice with pre-B-cell lymphoma, compared with the parent compound (P = 0.015). Interestingly, we also show that these HSP70 inhibitors impair the activity of the anaphase promoting complex/cyclosome (APC/C) in cell-free extracts, and induce G2-M arrest and genomic instability in cancer cells. PES-Cl is thus a promising new anticancer compound with several notable mechanisms of action.
Conflict of interest statement
The authors declare there are no conflicts of interest
Figures


References
-
- Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–10. - PubMed
-
- Powers MV, Jones K, Barillari C, Westwood I, van Montfort RL, Workman P. Targeting HSP70: the second potentially druggable heat shock protein and molecular chaperone? Cell Cycle. 2010;9:1542–50. - PubMed
-
- Schmitt E, Maingret L, Puig PE, Rerole AL, Ghiringhelli F, Hammann A, Solary E, Kroemer G, Garrido C. Heat shock protein 70 neutralization exerts potent antitumor effects in animal models of colon cancer and melanoma. Cancer Res. 2006;66:4191–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

