Bidirectional regulation of intravenous general anesthetic actions by α3-containing γ-aminobutyric acid A receptors
- PMID: 23303487
- PMCID: PMC3951843
- DOI: 10.1097/ALN.0b013e3182800d76
Bidirectional regulation of intravenous general anesthetic actions by α3-containing γ-aminobutyric acid A receptors
Abstract
Background: γ-aminobutyric acid A (GABAA) receptors mediate the actions of several intravenous general anesthetics. However, the contribution of α3-containing GABAA receptors to the action of these drugs is unknown.
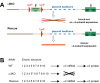
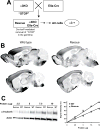
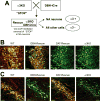
Methods: The authors compared anesthetic endpoints (hypnosis, immobility, hypothermia) in response to various intravenous anesthetics in mice lacking the α3 subunit of the GABAA receptor (α3 knockout) and in wild-type mice. Furthermore, the authors generated and analyzed conditional mutant mice expressing the GABAA receptor α3 subunit exclusively in noradrenergic neurons.
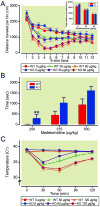
Results: α3 knockout mice displayed decreased hypnotic and hypothermic responses to etomidate and midazolam, but an increased response to pentobarbital. The hypnotic response to ketamine was unaltered, whereas the hypothermic response was increased. In contrast, the hypnotic but not the hypothermic response to medetomidine was increased. The combination of ketamine/xylazine displayed increased hypnotic, immobilizing, and hypothermic effects in α3 knockout mice. Mice expressing the α3 subunit exclusively in noradrenergic neurons were generated to assess whether the lack of α3 subunits on noradrenergic neurons may be responsible for this effect. In these mice, the increases of the hypnotic and immobilizing actions induced by ketamine/xylazine were largely absent, whereas the increase in the hypothermic action was still present.
Conclusion: α3-containing GABAA receptors bidirectionally regulate essential anesthetic actions: they mediate anesthetic actions of etomidate and midazolam, known to selectively act at GABAA receptors, and they negatively constrain anesthetic actions of compounds with targets partly or exclusively distinct from GABAA receptors such as medetomidine, ketamine, and pentobarbital. Furthermore, our results indicate that α3-containing GABAA receptors on noradrenergic neurons may contribute to this constraint.
Conflict of interest statement
Figures


Similar articles
-
Mapping the contribution of beta3-containing GABAA receptors to volatile and intravenous general anesthetic actions.BMC Pharmacol. 2007 Feb 24;7:2. doi: 10.1186/1471-2210-7-2. BMC Pharmacol. 2007. PMID: 17319964 Free PMC article.
-
General anesthetic actions on GABAA receptors in vivo are reduced in phospholipase C-related catalytically inactive protein knockout mice.J Anesth. 2017 Aug;31(4):531-538. doi: 10.1007/s00540-017-2350-2. Epub 2017 Apr 7. J Anesth. 2017. PMID: 28389811
-
Drug-selective Anesthetic Insensitivity of Zebrafish Lacking γ-Aminobutyric Acid Type A Receptor β3 Subunits.Anesthesiology. 2019 Dec;131(6):1276-1291. doi: 10.1097/ALN.0000000000002963. Anesthesiology. 2019. PMID: 31567362 Free PMC article.
-
Closing the gap between the molecular and systemic actions of anesthetic agents.Adv Pharmacol. 2015;72:229-62. doi: 10.1016/bs.apha.2014.10.009. Epub 2014 Dec 4. Adv Pharmacol. 2015. PMID: 25600373 Review.
-
[Roles of glutamatergic and GABAergic nervous system in hypnotic and analgesic actions of general anesthetics].Masui. 2011 May;60(5):534-43. Masui. 2011. PMID: 21626857 Review. Japanese.
Cited by
-
Itch suppression in mice and dogs by modulation of spinal α2 and α3GABAA receptors.Nat Commun. 2018 Aug 13;9(1):3230. doi: 10.1038/s41467-018-05709-0. Nat Commun. 2018. PMID: 30104684 Free PMC article.
-
SB-205384 is a positive allosteric modulator of recombinant GABAA receptors containing rat α3, α5, or α6 subunit subtypes coexpressed with β3 and γ2 subunits.J Pharmacol Exp Ther. 2013 Oct;347(1):235-41. doi: 10.1124/jpet.113.207324. Epub 2013 Jul 31. J Pharmacol Exp Ther. 2013. PMID: 23902941 Free PMC article.
-
GABA system as the cause and effect in early development.Neurosci Biobehav Rev. 2024 Jun;161:105651. doi: 10.1016/j.neubiorev.2024.105651. Epub 2024 Apr 4. Neurosci Biobehav Rev. 2024. PMID: 38579901 Free PMC article. Review.
References
-
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. - PubMed
-
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. - PubMed
-
- Bonin RP, Orser BA. GABAA receptor subtypes underlying general anesthesia. Pharmacol Biochem Behav. 2008;90:105–12. - PubMed
-
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J. 2003;17:250–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

