Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer
- PMID: 23303565
- PMCID: PMC3565104
- DOI: 10.1210/er.2012-1043
Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer
Abstract
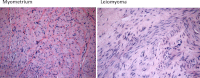
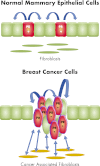
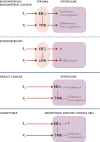
Progesterone receptor (PR) mediates the actions of the ovarian steroid progesterone, which together with estradiol regulates gonadotropin secretion, prepares the endometrium for implantation, maintains pregnancy, and differentiates breast tissue. Separation of estrogen and progesterone actions in hormone-responsive tissues remains a challenge. Pathologies of the uterus and breast, including endometrial cancer, endometriosis, uterine fibroids, and breast cancer, are highly associated with estrogen, considered to be the mitogenic factor. Emerging evidence supports distinct roles of progesterone and its influence on the pathogenesis of these diseases. Progesterone antagonizes estrogen-driven growth in the endometrium, and insufficient progesterone action strikingly increases the risk of endometrial cancer. In endometriosis, eutopic and ectopic tissues do not respond sufficiently to progesterone and are considered to be progesterone-resistant, which contributes to proliferation and survival. In uterine fibroids, progesterone promotes growth by increasing proliferation, cellular hypertrophy, and deposition of extracellular matrix. In normal mammary tissue and breast cancer, progesterone is pro-proliferative and carcinogenic. A key difference between these tissues that could explain the diverse effects of progesterone is the paracrine interactions of PR-expressing stroma and epithelium. Normal endometrium is a mucosa containing large quantities of distinct stromal cells with abundant PR, which influences epithelial cell proliferation and differentiation and protects against carcinogenic transformation. In contrast, the primary target cells of progesterone in the breast and fibroids are the mammary epithelial cells and the leiomyoma cells, which lack specifically organized stromal components with significant PR expression. This review provides a unifying perspective for the diverse effects of progesterone across human tissues and diseases.
Figures
References
-
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 - PubMed
-
- Graham JD, Clarke CL. 1997. Physiological action of progesterone in target tissues. Endocr Rev 18:502–519 - PubMed
-
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. 2000. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

