Human cartilage repair with a photoreactive adhesive-hydrogel composite
- PMID: 23303605
- PMCID: PMC3972413
- DOI: 10.1126/scitranslmed.3004838
Human cartilage repair with a photoreactive adhesive-hydrogel composite
Abstract
Surgical options for cartilage resurfacing may be significantly improved by advances and application of biomaterials that direct tissue repair. A poly(ethylene glycol) diacrylate (PEGDA) hydrogel was designed to support cartilage matrix production, with easy surgical application. A model in vitro system demonstrated deposition of cartilage-specific extracellular matrix in the hydrogel biomaterial and stimulation of adjacent cartilage tissue development by mesenchymal stem cells. For translation to the joint environment, a chondroitin sulfate adhesive was applied to covalently bond and adhere the hydrogel to cartilage and bone tissue in articular defects. After preclinical testing in a caprine model, a pilot clinical study was initiated where the biomaterials system was combined with standard microfracture surgery in 15 patients with focal cartilage defects on the medial femoral condyle. Control patients were treated with microfracture alone. Magnetic resonance imaging showed that treated patients achieved significantly higher levels of tissue fill compared to controls. Magnetic resonance spin-spin relaxation times (T(2)) showed decreasing water content and increased tissue organization over time. Treated patients had less pain compared with controls, whereas knee function [International Knee Documentation Committee (IKDC)] scores increased to similar levels between the groups over the 6 months evaluated. No major adverse events were observed over the study period. With further clinical testing, this practical biomaterials strategy has the potential to improve the treatment of articular cartilage defects.
Conflict of interest statement
Figures
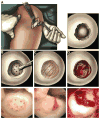


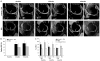
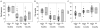
Comment in
-
Regenerative medicine: Adhesive-hydrogel composite developed to repair damaged cartilage.Nat Rev Rheumatol. 2013 Mar;9(3):135. doi: 10.1038/nrrheum.2013.9. Epub 2013 Jan 29. Nat Rev Rheumatol. 2013. PMID: 23358508 No abstract available.
References
-
- Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–2112. - PubMed
-
- Van Assche D, Staes F, Van Caspel D, Vanlauwe J, Bellemans J, Saris DB, Luyten FP. Autologous chondrocyte implantation versus microfracture for knee cartilage injury: A prospective randomized trial, with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:486–495. - PubMed
-
- Hunziker EB. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. - PubMed
-
- Custers RJ, Saris DB, Dhert WJ, Verbout AJ, van Rijen MH, Mastbergen SC, Lafeber FP, Creemers LB. Articular cartilage degeneration following the treatment of focal cartilage defects with ceramic metal implants and compared with microfracture. J Bone Joint Surg Am. 2009;91:900–910. - PubMed
-
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: An evidence-based systematic analysis. Am J Sports Med. 2009;37:2053–2063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

