Binding of circulating autoantibodies in breast cancer to native and peroxynitrite-modified RNA
- PMID: 23303630
- PMCID: PMC3542957
- DOI: 10.1631/jzus.B1200015
Binding of circulating autoantibodies in breast cancer to native and peroxynitrite-modified RNA
Abstract
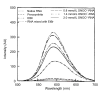
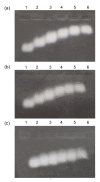
Peroxynitrite (ONOO(-)) is a powerful oxidant and nitrosative agent and has in vivo existence. The half life of ONOO(-) at physiological pH is less than 1 s. It can react with nucleic acids, proteins, lipoproteins, saccharides, cardiolipin, etc., and can modify their native structures. Action of ONOO(-), synthesized in the authors' laboratory by a rapid quenched flow process, on structural changes of commercially available RNA was studied by ultraviolet (UV), fluorescence, and agarose gel electrophoresis. Compared to native RNA, the ONOO(-)-modified RNA showed hyperchromicity at 260 nm. Furthermore, the ethidium bromide (EtBr) assisted emission intensities of ONOO(-)-modified RNA samples were found to be lower than the emission intensity of native RNA-EtBr complex. Agarose gel electrophoresis of ONOO(-)-modified RNA showed a gradual decrease in band intensities compared to native RNA, an observation clearly due to the poor intercalation of EtBr with ONOO(-)-modified RNA. Native and ONOO(-)-modified RNA samples were used as an antigen to detect autoantibodies in sera of patients with clinically defined breast cancer. Both direct binding and inhibition enzyme-linked immunosorbent assay (ELISA) confirmed the prevalence of native and 0.8 mmol/L ONOO(-)-modified RNA specific autoantibodies in breast cancer patients. Moreover, the progressive retardation in the mobility of immune complexes formed with native or 0.8 mmol/L ONOO(-)-modified RNA and affinity purified immunoglobulin G (IgG) from sera of breast cancer patients supports the findings of the direct binding and inhibition ELISAs. The peroxynitrite treatment to RNA at a higher concentration appears to have damaged or destroyed the typical epitopes on RNA and thus there was a sharp decrease in autoantibodies binding to 1.4 mmol/L ONOO(-)-modified RNA. It may be interpreted that cellular nitrosative stress can modify and confer immunogenicity on RNA molecules. Higher concentrations of nitrogen reactive species can be detrimental to RNA. However, the emergence of native as well as 0.8 mmol/L ONOO(-)-modified RNA as a novel antigen/substrate for autoantibodies in breast cancer patients indicates that, in future, these molecules might find a place on the panel of antigens for early diagnosis of breast cancer.
Figures


Similar articles
-
Physicochemical and immunological studies on mitochondrial DNA modified by peroxynitrite: implications of neo-epitopes of mitochondrial DNA in the etiopathogenesis of systemic lupus erythematosus.Lupus. 2013 Sep;22(10):1024-37. doi: 10.1177/0961203313498803. Epub 2013 Jul 24. Lupus. 2013. PMID: 23884988
-
Peroxynitrite modified DNA may be an antigenic trigger for antibodies in various cancers of gynecologic origin.Hum Immunol. 2013 Oct;74(10):1239-43. doi: 10.1016/j.humimm.2013.07.015. Epub 2013 Aug 2. Hum Immunol. 2013. PMID: 23911359
-
Immunological studies on peroxynitrite modified human DNA.Life Sci. 2005 Oct 7;77(21):2626-42. doi: 10.1016/j.lfs.2005.02.026. Life Sci. 2005. PMID: 16098994
-
Peroxynitrite: cellular pathology and implications in autoimmunity.J Immunoassay Immunochem. 2019;40(2):123-138. doi: 10.1080/15321819.2019.1583109. Epub 2019 Mar 7. J Immunoassay Immunochem. 2019. PMID: 30843753 Review.
-
[Role of peroxynitrite anion in different diseases].Rev Invest Clin. 2006 Jul-Aug;58(4):350-8. Rev Invest Clin. 2006. PMID: 17146946 Review. Spanish.
References
-
- Dixit K, Khan MA, Sharma YD, Moinuddin , Alam K. Peroxynitrite-induced modification of H2A histone presents epitopes which are strongly bound by human ant-DNA autoantibodies: role of peroxynitrite-modified-H2A in SLE induction and progression. Hum Immunol. 2011;72(3):219–225. doi: 10.1016/j.humimm.2010.12.004. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

