Primary melanoma of the CNS in children is driven by congenital expression of oncogenic NRAS in melanocytes
- PMID: 23303902
- PMCID: PMC3999382
- DOI: 10.1158/2159-8290.CD-12-0464
Primary melanoma of the CNS in children is driven by congenital expression of oncogenic NRAS in melanocytes
Abstract
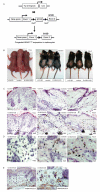
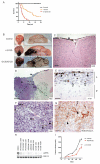
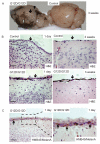
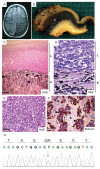
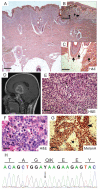
NRAS mutations are common in human melanoma. To produce a mouse model of NRAS-driven melanoma, we expressed oncogenic NRAS (NRAS(G12D)) in mouse melanocytes. When NRAS(G12D) was expressed in the melanocytes of developing embryos, it induced melanocyte proliferation and congenital melanocytic lesions reminiscent of human blue nevi but did not induce cutaneous melanoma. Unexpectedly, however, it did induce early-onset primary melanoma of the central nervous system (CNS). The tumors were rapidly proliferating and caused neurologic symptoms, rapid health deterioration, and death. NRAS is not a common driver oncogene of primary melanoma of the CNS in adults, but we report two cases of primary melanoma of the CNS in children, both of which carried oncogenic mutations in NRAS. We conclude that acquisition of somatic mutations in NRAS in CNS melanocytes is a predisposing risk factor for primary melanoma of the CNS in children, and we present a mouse model of this disease.
Significance: We show that the acquisition of NRAS mutations in melanocytes during embryogenesis is a risk factor for early-onset melanoma of the CNS. We have developed a powerful mouse model to study this rare but devastating childhood disease, and to develop therapeutic approaches for its treatment.
©2013 AACR.
Figures
Comment in
-
A model for primary melanoma of the CNS implicates NRAS.Cancer Discov. 2013 Apr;3(4):382-3. doi: 10.1158/2159-8290.CD-13-0076. Cancer Discov. 2013. PMID: 23580282
Similar articles
-
A model for primary melanoma of the CNS implicates NRAS.Cancer Discov. 2013 Apr;3(4):382-3. doi: 10.1158/2159-8290.CD-13-0076. Cancer Discov. 2013. PMID: 23580282
-
Activating mutations of the GNAQ gene: a frequent event in primary melanocytic neoplasms of the central nervous system.Acta Neuropathol. 2010 Mar;119(3):317-23. doi: 10.1007/s00401-009-0611-3. Acta Neuropathol. 2010. PMID: 19936769 Free PMC article.
-
Neural crest state activation in NRAS driven melanoma, but not in NRAS-driven melanocyte expansion.Dev Biol. 2019 May 15;449(2):107-114. doi: 10.1016/j.ydbio.2018.05.026. Epub 2018 Jun 5. Dev Biol. 2019. PMID: 29883661 Free PMC article.
-
Primary melanocytic tumors of the central nervous system: a review with focus on molecular aspects.Brain Pathol. 2015 Mar;25(2):209-26. doi: 10.1111/bpa.12241. Brain Pathol. 2015. PMID: 25534128 Free PMC article. Review.
-
BRAF and NRAS mutations are uncommon in melanomas arising in diverse internal organs.J Clin Pathol. 2005 Jun;58(6):640-4. doi: 10.1136/jcp.2004.022509. J Clin Pathol. 2005. PMID: 15917418 Free PMC article. Review.
Cited by
-
Primary Meningeal Melanocytic Tumors of the Central Nervous System: A Review from the Ultra-Rare Brain Tumors Task Force of the European Network for Rare Cancers (EURACAN).Cancers (Basel). 2024 Jul 10;16(14):2508. doi: 10.3390/cancers16142508. Cancers (Basel). 2024. PMID: 39061148 Free PMC article. Review.
-
Missense mutation of NRAS is associated with malignant progression in neurocutaneous melanosis.Acta Neuropathol Commun. 2024 Jan 22;12(1):14. doi: 10.1186/s40478-024-01723-0. Acta Neuropathol Commun. 2024. PMID: 38254245 Free PMC article.
-
Mutation-specific RAS oncogenicity explains NRAS codon 61 selection in melanoma.Cancer Discov. 2014 Dec;4(12):1418-29. doi: 10.1158/2159-8290.CD-14-0729. Epub 2014 Sep 24. Cancer Discov. 2014. PMID: 25252692 Free PMC article.
-
Current State of Animal (Mouse) Modeling in Melanoma Research.Cancer Growth Metastasis. 2015 Oct 6;8(Suppl 1):81-94. doi: 10.4137/CGM.S21214. eCollection 2015. Cancer Growth Metastasis. 2015. PMID: 26483610 Free PMC article. Review.
-
Pericyte Ontogeny: The Use of Chimeras to Track a Cell Lineage of Diverse Germ Line Origins.Methods Mol Biol. 2021;2235:61-87. doi: 10.1007/978-1-0716-1056-5_6. Methods Mol Biol. 2021. PMID: 33576971
References
-
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. - PubMed
-
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664–78. - PubMed
-
- Seddon JM, Gragoudas ES, Glynn RJ, Egan KM, Albert DM, Blitzer PH. Host factors, UV radiation, and risk of uveal melanoma. A case-control study. Arch Ophthalmol. 1990;108:1274–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

