An evolutionarily conserved signaling mechanism mediates far-red light responses in land plants
- PMID: 23303916
- PMCID: PMC3584528
- DOI: 10.1105/tpc.112.104331
An evolutionarily conserved signaling mechanism mediates far-red light responses in land plants
Erratum in
-
Correction.Plant Cell. 2015 Jul;27(7):2075. doi: 10.1105/tpc.15.00527. Epub 2015 Jun 19. Plant Cell. 2015. PMID: 26091692 Free PMC article. No abstract available.
Abstract
Phytochromes are plant photoreceptors important for development and adaptation to the environment. Phytochrome A (PHYA) is essential for the far-red (FR) high-irradiance responses (HIRs), which are of particular ecological relevance as they enable plants to establish under shade conditions. PHYA and HIRs have been considered unique to seed plants because the divergence of seed plants and cryptogams (e.g., ferns and mosses) preceded the evolution of PHYA. Seed plant phytochromes translocate into the nucleus and regulate gene expression. By contrast, there has been little evidence of a nuclear localization and function of cryptogam phytochromes. Here, we identified responses to FR light in cryptogams, which are highly reminiscent of PHYA signaling in seed plants. In the moss Physcomitrella patens and the fern Adiantum capillus-veneris, phytochromes accumulate in the nucleus in response to light. Although P. patens phytochromes evolved independently of PHYA, we have found that one clade of P. patens phytochromes exhibits the molecular properties of PHYA. We suggest that HIR-like responses had evolved in the last common ancestor of modern seed plants and cryptogams and that HIR signaling is more ancient than PHYA. Thus, other phytochromes in seed plants may have lost the capacity to mediate HIRs during evolution, rather than that PHYA acquired it.
Figures
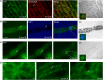
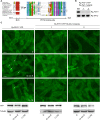
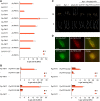
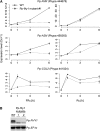

Similar articles
-
Missense mutation in the amino terminus of phytochrome A disrupts the nuclear import of the photoreceptor.Plant Physiol. 2012 Jan;158(1):107-18. doi: 10.1104/pp.111.186288. Epub 2011 Oct 10. Plant Physiol. 2012. PMID: 21969386 Free PMC article.
-
Shedding (far-red) light on phytochrome mechanisms and responses in land plants.Plant Sci. 2014 Mar;217-218:36-46. doi: 10.1016/j.plantsci.2013.11.013. Epub 2013 Nov 28. Plant Sci. 2014. PMID: 24467894 Review.
-
Arabidopsis phytochrome a is modularly structured to integrate the multiple features that are required for a highly sensitized phytochrome.Plant Cell. 2012 Jul;24(7):2949-62. doi: 10.1105/tpc.111.094201. Epub 2012 Jul 27. Plant Cell. 2012. PMID: 22843485 Free PMC article.
-
Photoreceptor partner FHY1 has an independent role in gene modulation and plant development under far-red light.Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):11888-93. doi: 10.1073/pnas.1412528111. Epub 2014 Jul 28. Proc Natl Acad Sci U S A. 2014. PMID: 25071219 Free PMC article.
-
Two Distinct Molecular Types of Phytochrome A in Plants: Evidence of Existence and Implications for Functioning.Int J Mol Sci. 2023 May 2;24(9):8139. doi: 10.3390/ijms24098139. Int J Mol Sci. 2023. PMID: 37175844 Free PMC article. Review.
Cited by
-
Streptophyte phytochromes exhibit an N-terminus of cyanobacterial origin and a C-terminus of proteobacterial origin.BMC Res Notes. 2015 Apr 13;8:144. doi: 10.1186/s13104-015-1082-3. BMC Res Notes. 2015. PMID: 25886068 Free PMC article.
-
Pseudocrossidium replicatum (Taylor) R.H. Zander is a fully desiccation-tolerant moss that expresses an inducible molecular mechanism in response to severe abiotic stress.Plant Mol Biol. 2021 Nov;107(4-5):387-404. doi: 10.1007/s11103-021-01167-3. Epub 2021 Jun 29. Plant Mol Biol. 2021. PMID: 34189708 Free PMC article.
-
A small and highly sensitive red/far-red optogenetic switch for applications in mammals.Nat Biotechnol. 2022 Feb;40(2):262-272. doi: 10.1038/s41587-021-01036-w. Epub 2021 Oct 4. Nat Biotechnol. 2022. PMID: 34608325
-
Arabidopsis FHY1 and FHY1-LIKE Are Not Required for Phytochrome A Signal Transduction in the Nucleus.Plant Commun. 2019 Nov 9;1(2):100007. doi: 10.1016/j.xplc.2019.100007. eCollection 2020 Mar 9. Plant Commun. 2019. PMID: 33404546 Free PMC article.
-
Phytochrome Coordinates with a hnRNP to Regulate Alternative Splicing via an Exonic Splicing Silencer.Plant Physiol. 2020 Jan;182(1):243-254. doi: 10.1104/pp.19.00289. Epub 2019 Sep 9. Plant Physiol. 2020. PMID: 31501299 Free PMC article.
References
-
- Burgin M.J., Casal J.J., Whitelam G.C., Sanchez R.A. (1999). A light-regulated pool of phytochrome and rudimentary high-irradiance responses under far-red light in Pinus elliottii and Pseudotsuga menziesii. J. Exp. Bot. 50: 831–836
-
- Chen M., Tao Y., Lim J., Shaw A., Chory J. (2005). Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr. Biol. 15: 637–642 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

