Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals
- PMID: 23303926
- PMCID: PMC3711765
- DOI: 10.1523/JNEUROSCI.2136-12.2013
Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals
Abstract
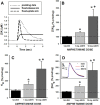
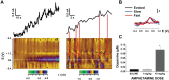
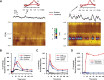
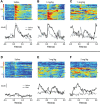
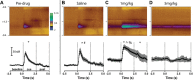
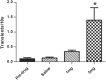
Drugs of abuse hijack brain-reward circuitry during the addiction process by augmenting action potential-dependent phasic dopamine release events associated with learning and goal-directed behavior. One prominent exception to this notion would appear to be amphetamine (AMPH) and related analogs, which are proposed instead to disrupt normal patterns of dopamine neurotransmission by depleting vesicular stores and promoting nonexocytotic dopamine efflux via reverse transport. This mechanism of AMPH action, though, is inconsistent with its therapeutic effects and addictive properties, which are thought to be reliant on phasic dopamine signaling. Here we used fast-scan cyclic voltammetry in freely moving rats to interrogate principal neurochemical responses to AMPH in the striatum and relate these changes to behavior. First, we showed that AMPH dose-dependently enhanced evoked dopamine responses to phasic-like current pulse trains for up to 2 h. Modeling the data revealed that AMPH inhibited dopamine uptake but also unexpectedly potentiated vesicular dopamine release. Second, we found that AMPH increased the amplitude, duration, and frequency of spontaneous dopamine transients, the naturally occurring, nonelectrically evoked, phasic increases in extracellular dopamine. Finally, using an operant sugar reward paradigm, we showed that low-dose AMPH augmented dopamine transients elicited by sugar-predictive cues. However, operant behavior failed at high-dose AMPH, which was due to phasic dopamine hyperactivity and the decoupling of dopamine transients from the reward predictive cue. These findings identify upregulation of exocytotic dopamine release as a key AMPH action in behaving animals and support a unified mechanism of abused drugs to activate phasic dopamine signaling.
Figures


Comment in
-
Amphetamine mechanisms and actions at the dopamine terminal revisited.J Neurosci. 2013 May 22;33(21):8923-5. doi: 10.1523/JNEUROSCI.1033-13.2013. J Neurosci. 2013. PMID: 23699503 Free PMC article. No abstract available.
References
-
- Anderson BB, Chen G, Gutman DA, Ewing AG. Dopamine levels of two classes of vesicles are differentially depleted by amphetamine. Brain Res. 1998;788:294–301. - PubMed
-
- Bowyer JF, Masserano JM, Weiner N. Inhibitory effects of amphetamine on potassium-stimulated release of [3H]dopamine from striatal slices and synaptosomes. J Pharmacol Exp Ther. 1987;240:177–186. - PubMed
-
- Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–348. - PubMed
-
- Chadchankar H, Ihalainen J, Tanila H, Yavich L. Methylphenidate modifies overflow and presynaptic compartmentalization of dopamine via an alpha-synuclein-dependent mechanism. J Pharmacol Exp Ther. 2012;341:484–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
