Melanocortin-4 receptor regulates hippocampal synaptic plasticity through a protein kinase A-dependent mechanism
- PMID: 23303927
- PMCID: PMC6704894
- DOI: 10.1523/JNEUROSCI.3282-12.2013
Melanocortin-4 receptor regulates hippocampal synaptic plasticity through a protein kinase A-dependent mechanism
Abstract
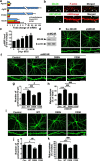
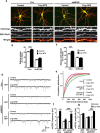
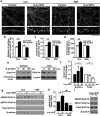
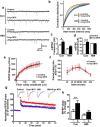
Learning and memory require orchestrated regulation of both structural and functional synaptic plasticity in the hippocampus. While a neuropeptide alpha-melanocyte-stimulating hormone, α-MSH, has been implicated in memory acquisition and retention, the functional role of its cognate receptor, melanocortin-4 receptor (MC4R), in hippocampal-dependent synaptic plasticity has not been explored. In this study, we report that activation of MC4R enhances synaptic plasticity through the regulation of dendritic spine morphology and abundance of AMPA receptors. We show that activation of postsynaptic MC4R increases the number of mature dendritic spines and enhances surface expression of AMPA receptor subunit GluA1, resulting in synaptic accumulation of GluA1-containing AMPA receptors. Moreover, MC4R stimulates surface GluA1 trafficking through phosphorylation of GluA1 at Ser845 in a Gα(s)-cAMP/PKA-dependent manner. Blockade of protein kinase A (PKA) signaling abolishes the MC4R-mediated enhancement of neurotransmission and hippocampal long-term potentiation. Importantly, in vivo application of MC4R agonists increases LTP in the mouse hippocampal CA1 region. These findings reveal that MC4R in the hippocampus plays a critical role in the regulation of structural and functional plasticity.
Figures

Similar articles
-
Control of Homeostatic Synaptic Plasticity by AKAP-Anchored Kinase and Phosphatase Regulation of Ca2+-Permeable AMPA Receptors.J Neurosci. 2018 Mar 14;38(11):2863-2876. doi: 10.1523/JNEUROSCI.2362-17.2018. Epub 2018 Feb 13. J Neurosci. 2018. PMID: 29440558 Free PMC article.
-
PrPC controls via protein kinase A the direction of synaptic plasticity in the immature hippocampus.J Neurosci. 2013 Feb 13;33(7):2973-83. doi: 10.1523/JNEUROSCI.4149-12.2013. J Neurosci. 2013. PMID: 23407955 Free PMC article.
-
Stimulation of the Hippocampal POMC/MC4R Circuit Alleviates Synaptic Plasticity Impairment in an Alzheimer's Disease Model.Cell Rep. 2016 Nov 8;17(7):1819-1831. doi: 10.1016/j.celrep.2016.10.043. Cell Rep. 2016. PMID: 27829153
-
Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases.Prog Neurobiol. 2003 Dec;71(6):401-37. doi: 10.1016/j.pneurobio.2003.12.003. Prog Neurobiol. 2003. PMID: 15013227 Review.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
Cited by
-
Axin Regulates Dendritic Spine Morphogenesis through Cdc42-Dependent Signaling.PLoS One. 2015 Jul 23;10(7):e0133115. doi: 10.1371/journal.pone.0133115. eCollection 2015. PLoS One. 2015. PMID: 26204446 Free PMC article.
-
Evidence for phenotypic plasticity in response to photic cues and the connection with genes of risk in schizophrenia.Front Behav Neurosci. 2013 Jul 9;7:82. doi: 10.3389/fnbeh.2013.00082. eCollection 2013. Front Behav Neurosci. 2013. PMID: 23847488 Free PMC article.
-
MC4R Localizes at Excitatory Postsynaptic and Peri-Postsynaptic Sites of Hypothalamic Neurons in Primary Culture.Cells. 2024 Jul 23;13(15):1235. doi: 10.3390/cells13151235. Cells. 2024. PMID: 39120267 Free PMC article.
-
Reactive oxygen species generation mediated by NADPH oxidase and PI3K/Akt pathways contribute to invasion of Streptococcus agalactiae in human endothelial cells.Mem Inst Oswaldo Cruz. 2018;113(6):e140421. doi: 10.1590/0074-02760170421. Epub 2018 Apr 5. Mem Inst Oswaldo Cruz. 2018. PMID: 29641644 Free PMC article.
-
Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake.Nat Commun. 2016 Jan 8;7:10268. doi: 10.1038/ncomms10268. Nat Commun. 2016. PMID: 26743492 Free PMC article.
References
-
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. - PubMed
-
- Bito H, Takemoto-Kimura S. Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium. 2003;34:425–430. - PubMed
-
- Cheung ZH, Ip NY. From understanding synaptic plasticity to the development of cognitive enhancers. Int J Neuropsychopharmacol. 2011;14:1247–1256. - PubMed
-
- Chklovskii DB. Synaptic connectivity and neuronal morphology: two sides of the same coin. Neuron. 2004;43:609–617. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
