Physostigmine and galanthamine bind in the presence of agonist at the canonical and noncanonical subunit interfaces of a nicotinic acetylcholine receptor
- PMID: 23303929
- PMCID: PMC3542985
- DOI: 10.1523/JNEUROSCI.3483-12.2013
Physostigmine and galanthamine bind in the presence of agonist at the canonical and noncanonical subunit interfaces of a nicotinic acetylcholine receptor
Abstract
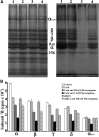
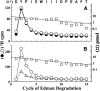
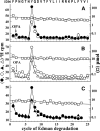
Galanthamine and physostigmine are clinically used cholinomimetics that both inhibit acetylcholinesterase and also interact directly with and potentiate nAChRs. As with most nAChR-positive allosteric modulators, the location and number of their binding site(s) within nAChRs are unknown. In this study, we use the intrinsic photoreactivities of [(3)H]physostigmine and [(3)H]galanthamine upon irradiation at 312 nm to directly identify amino acids contributing to their binding sites in the Torpedo californica nAChR. Protein sequencing of fragments isolated from proteolytic digests of [(3)H]physostigmine- or [(3)H]galanthamine-photolabeled nAChR establish that, in the presence of agonist (carbamylcholine), both drugs photolabeled amino acids on the complementary (non-α) surface of the transmitter binding sites (γTyr-111/γTyr-117/δTyr172). They also photolabeled δTyr-212 at the δ-β subunit interface and γTyr-105 in the vestibule of the ion channel, with photolabeling of both residues enhanced in the presence of agonist. Furthermore, [(3)H]physostigmine photolabeling of γTyr-111, γTyr-117, δTyr-212, and γTyr-105 was inhibited in the presence of nonradioactive galanthamine. The locations of the photolabeled amino acids in the nAChR structure and the results of computational docking studies provide evidence that, in the presence of agonist, physostigmine and galanthamine bind to at least three distinct sites in the nAChR extracellular domain: at the α-γ interface (1) in the entry to the transmitter binding site and (2) in the vestibule of the ion channel near the level of the transmitter binding site, and at the δ-β interface (3) in a location equivalent to the benzodiazepine binding site in GABA(A) receptors.
Figures




References
-
- Arias HR. Positive and negative modulation of nicotinic receptors. Adv Prot Chem Struct Biol. 2010;80:153–203. - PubMed
-
- Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–1163. - PubMed
-
- Blanton MP, Cohen JB. Identifying the lipid-protein interface of the Torpedo nicotinic acetylcholine receptor: secondary structure implications. Biochemistry. 1994;33:2859–2872. - PubMed
-
- Brauer AW, Oman CL, Margolies MN. Use of ophthalaldehyde to reduce background during automated Edman degradation. Anal Biochem. 1984;137:134–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
