Gestational stress promotes pathological apneas and sex-specific disruption of respiratory control development in newborn rat
- PMID: 23303936
- PMCID: PMC6704897
- DOI: 10.1523/JNEUROSCI.1214-12.2013
Gestational stress promotes pathological apneas and sex-specific disruption of respiratory control development in newborn rat
Abstract
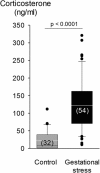
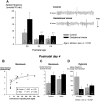
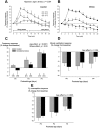
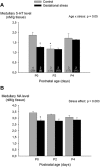
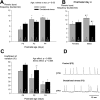
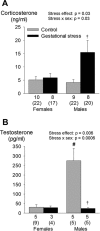
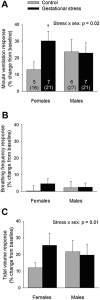
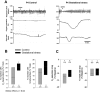
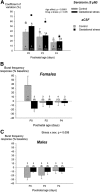
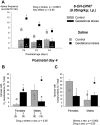
Recurrent apneas are important causes of hospitalization and morbidity in newborns. Gestational stress (GS) compromises fetal brain development. Maternal stress and anxiety during gestation are linked to respiratory disorders in newborns; however, the mechanisms remain unknown. Here, we tested the hypothesis that repeated activation of the neuroendocrine response to stress during gestation is sufficient to disrupt the development of respiratory control and augment the occurrence of apneas in newborn rats. Pregnant dams were displaced and exposed to predator odor from days 9 to 19 of gestation. Control dams were undisturbed. Experiments were performed on male and female rats aged between 0 and 4 d old. Apnea frequency decreased with age but was consistently higher in stressed pups than controls. At day 4, GS augmented the proportion of apneas with O(2) desaturations by 12%. During acute hypoxia (12% O(2)), the reflexive increase in breathing augmented with age; however, this response was lower in stressed pups. Instability of respiratory rhythm recorded from medullary preparations decreased with age but was higher in stressed pups than controls. GS reduced medullary serotonin (5-HT) levels in newborn pups by 32%. Bath application of 5-HT and injection of 8-OH-DPAT [(±)-8-hydroxy-2-di-(n-propylamino) tetralin hydrobromide; 5-HT(1A) agonist; in vivo] reduced respiratory instability and apneas; these effects were greater in stressed pups than controls. Sex-specific effects were observed. We conclude that activation of the stress response during gestation is sufficient to disrupt respiratory control development and promote pathological apneas in newborn rats. A deficit in medullary 5-HT contributes to these effects.
Figures
Similar articles
-
Sex-Specific Consequences of Neonatal Stress on Cardio-Respiratory Inhibition Following Laryngeal Stimulation in Rat Pups.eNeuro. 2018 Jan 4;4(6):ENEURO.0393-17.2017. doi: 10.1523/ENEURO.0393-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2018. PMID: 29308430 Free PMC article.
-
Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats.J Physiol. 2005 Jul 15;566(Pt 2):543-57. doi: 10.1113/jphysiol.2005.083873. Epub 2005 May 5. J Physiol. 2005. PMID: 15878953 Free PMC article.
-
Neonatal stress increases respiratory instability in rat pups.Respir Physiol Neurobiol. 2011 May 31;176(3):103-9. doi: 10.1016/j.resp.2011.01.014. Epub 2011 Feb 16. Respir Physiol Neurobiol. 2011. PMID: 21310268
-
Serotonin neurons and central respiratory chemoreception: where are we now?Prog Brain Res. 2014;209:207-33. doi: 10.1016/B978-0-444-63274-6.00011-4. Prog Brain Res. 2014. PMID: 24746050 Free PMC article. Review.
-
Gestational intermittent hypoxia increases susceptibility to neuroinflammation and alters respiratory motor control in neonatal rats.Respir Physiol Neurobiol. 2018 Oct;256:128-142. doi: 10.1016/j.resp.2017.11.007. Epub 2017 Nov 22. Respir Physiol Neurobiol. 2018. PMID: 29174411 Free PMC article. Review.
Cited by
-
Sex-Specific Consequences of Neonatal Stress on Cardio-Respiratory Inhibition Following Laryngeal Stimulation in Rat Pups.eNeuro. 2018 Jan 4;4(6):ENEURO.0393-17.2017. doi: 10.1523/ENEURO.0393-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2018. PMID: 29308430 Free PMC article.
-
Chronic prenatal stress epigenetically modifies spinal cord BDNF expression to induce sex-specific visceral hypersensitivity in offspring.Neurogastroenterol Motil. 2014 May;26(5):715-30. doi: 10.1111/nmo.12326. Epub 2014 Mar 4. Neurogastroenterol Motil. 2014. PMID: 24588943 Free PMC article.
-
Effects of gestational intermittent hypoxia on the respiratory system: A tale of the placenta, fetus, and developing offspring.J Sleep Res. 2025 Aug;34(4):e14435. doi: 10.1111/jsr.14435. Epub 2024 Dec 15. J Sleep Res. 2025. PMID: 39675784 Free PMC article. Review.
-
Disruption of estradiol regulation of orexin neurons: a novel mechanism in excessive ventilatory response to CO2 inhalation in a female rat model of panic disorder.Transl Psychiatry. 2020 Nov 10;10(1):394. doi: 10.1038/s41398-020-01076-x. Transl Psychiatry. 2020. PMID: 33173029 Free PMC article.
-
Sex- and Region-Specific Differences in the Transcriptomes of Rat Microglia from the Brainstem and Cervical Spinal Cord.J Pharmacol Exp Ther. 2020 Oct;375(1):210-222. doi: 10.1124/jpet.120.266171. Epub 2020 Jul 13. J Pharmacol Exp Ther. 2020. PMID: 32661056 Free PMC article.
References
-
- Al-Matary A, Kutbi I, Qurashi M, Khalil M, Alvaro R, Kwiatkowski K, Cates D, Rigatto H. Increased peripheral chemoreceptor activity may be critical in destabilizing breathing in neonates. Semin Perinatol. 2004;28:264–272. - PubMed
-
- Azmitia EC, Jr, McEwen BS. Adrenalcortical influence on rat brain tryptophan hydroxylase activity. Brain Res. 1974;78:291–302. - PubMed
-
- Baird TM. Clinical correlates, natural history and outcome of neonatal apnoea. Semin Neonatol. 2004;9:205–211. - PubMed
-
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1391–R1400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
