Enhanced recruitment of endosomal Na+/H+ exchanger NHE6 into Dendritic spines of hippocampal pyramidal neurons during NMDA receptor-dependent long-term potentiation
- PMID: 23303939
- PMCID: PMC6704919
- DOI: 10.1523/JNEUROSCI.2583-12.2013
Enhanced recruitment of endosomal Na+/H+ exchanger NHE6 into Dendritic spines of hippocampal pyramidal neurons during NMDA receptor-dependent long-term potentiation
Abstract
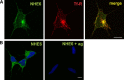
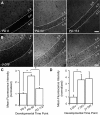
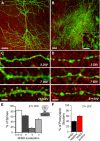
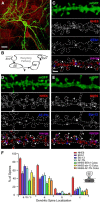
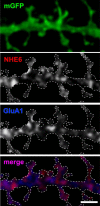
Postsynaptic endosomal trafficking has emerged as a principal regulatory mechanism of structural and functional plasticity of glutamatergic synapses. Recycling endosomes perform activity-dependent transport of AMPA receptors (AMPARs) and lipids to the postsynaptic membrane, activities that are known to contribute to long-term synaptic potentiation and hypothesized to subserve learning and memory processes in the brain. Recently, genetic defects in a widely expressed vesicular pH-regulating transporter, the Na(+)/H(+) exchanger NHE6 isoform, have been implicated in neurodevelopmental disorders including severe X-linked mental retardation and autism. However, little information is available regarding the cellular properties of this transporter in the CNS. Here, we show by quantitative light microscopy that the protein abundance of NHE6 is developmentally regulated in area CA1 of the mouse hippocampus. Within pyramidal neurons, NHE6 was found to localize to discrete puncta throughout the soma and neurites, with noticeable accumulation at dendritic spines and presynaptic terminals. Dual immunolabeling of dendritic spines revealed that NHE6 partially colocalizes with typical markers of early and recycling endosomes as well as with the AMPAR subunit GluA1. Significantly, NHE6-containing vesicles exhibited enhanced translocation to dendritic spine heads during NMDA receptor (NMDAR)-dependent long-term potentiation. These data suggest that NHE6 may play a unique, previously unrecognized, role at glutamatergic synapses that are important for learning and memory.
Figures


Similar articles
-
Impaired hippocampal plasticity associated with loss of recycling endosomal SLC9A6/NHE6 is ameliorated by the TrkB agonist 7,8-dihydroxyflavone.Biochim Biophys Acta Mol Basis Dis. 2025 Jan;1871(1):167529. doi: 10.1016/j.bbadis.2024.167529. Epub 2024 Sep 27. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39341363
-
A Christianson syndrome-linked deletion mutation (Δ287ES288) in SLC9A6 impairs hippocampal neuronal plasticity.Neurobiol Dis. 2019 Oct;130:104490. doi: 10.1016/j.nbd.2019.104490. Epub 2019 Jun 6. Neurobiol Dis. 2019. PMID: 31175985
-
Redistribution of microtubules in dendrites of hippocampal CA1 neurons after tetanic stimulation during long-term potentiation.Ital J Anat Embryol. 2008 Jan-Mar;113(1):17-27. Ital J Anat Embryol. 2008. PMID: 18491451
-
New insights in endosomal dynamics and AMPA receptor trafficking.Semin Cell Dev Biol. 2011 Jul;22(5):499-505. doi: 10.1016/j.semcdb.2011.06.008. Epub 2011 Aug 6. Semin Cell Dev Biol. 2011. PMID: 21843653 Review.
-
The role of endosomal-recycling in long-term potentiation.Cell Mol Life Sci. 2011 Jan;68(2):185-94. doi: 10.1007/s00018-010-0516-2. Epub 2010 Sep 6. Cell Mol Life Sci. 2011. PMID: 20820847 Free PMC article. Review.
Cited by
-
Endosomal pH in neuronal signaling and synaptic transmission: role of Na(+)/H(+) exchanger NHE5.Front Physiol. 2014 Jan 13;4:412. doi: 10.3389/fphys.2013.00412. eCollection 2014 Jan 13. Front Physiol. 2014. PMID: 24454292 Free PMC article. Review.
-
Loss of SLC9A6/NHE6 impairs nociception in a mouse model of Christianson syndrome.Pain. 2020 Nov;161(11):2619-2628. doi: 10.1097/j.pain.0000000000001961. Pain. 2020. PMID: 32569089 Free PMC article.
-
Mixed Neurodevelopmental and Neurodegenerative Pathology in Nhe6-Null Mouse Model of Christianson Syndrome.eNeuro. 2018 Jan 17;4(6):ENEURO.0388-17.2017. doi: 10.1523/ENEURO.0388-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2018. PMID: 29349289 Free PMC article.
-
Dynamics of nascent and active zone ultrastructure as synapses enlarge during long-term potentiation in mature hippocampus.J Comp Neurol. 2014 Dec 1;522(17):3861-84. doi: 10.1002/cne.23646. Epub 2014 Jul 30. J Comp Neurol. 2014. PMID: 25043676 Free PMC article.
-
X-linked Christianson syndrome: heterozygous female Slc9a6 knockout mice develop mosaic neuropathological changes and related behavioral abnormalities.Dis Model Mech. 2016 Jan;9(1):13-23. doi: 10.1242/dmm.022780. Epub 2015 Oct 29. Dis Model Mech. 2016. PMID: 26515654 Free PMC article.
References
-
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. - PubMed
-
- Brett CL, Wei Y, Donowitz M, Rao R. Human Na+/H+ exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol. 2002;282:C1031–C1041. - PubMed
-
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA Receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA Receptors during hippocampal LTD. Neuron. 2005;45:81–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
