Synaptic plasticity at intrathalamic connections via CaV3.3 T-type Ca2+ channels and GluN2B-containing NMDA receptors
- PMID: 23303941
- PMCID: PMC6704895
- DOI: 10.1523/JNEUROSCI.3185-12.2013
Synaptic plasticity at intrathalamic connections via CaV3.3 T-type Ca2+ channels and GluN2B-containing NMDA receptors
Abstract
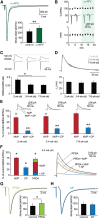
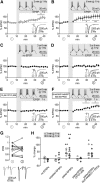
The T-type Ca(2+) channels encoded by the Ca(V)3 genes are well established electrogenic drivers for burst discharge. Here, using Ca(V)3.3(-/-) mice we found that Ca(V)3.3 channels trigger synaptic plasticity in reticular thalamic neurons. Burst discharge via Ca(V)3.3 channels induced long-term potentiation at thalamoreticular inputs when coactivated with GluN2B-containing NMDA receptors, which are the dominant subtype at these synapses. Notably, oscillatory burst discharge of reticular neurons is typical for sleep-related rhythms, suggesting that sleep contributes to strengthening intrathalamic circuits.
Figures


Similar articles
-
Impaired transmission at corticothalamic excitatory inputs and intrathalamic GABAergic synapses in the ventrobasal thalamus of heterozygous BDNF knockout mice.Neuroscience. 2012 Oct 11;222:215-27. doi: 10.1016/j.neuroscience.2012.07.005. Epub 2012 Jul 13. Neuroscience. 2012. PMID: 22796079
-
Synaptic organization and input-specific short-term plasticity in anterior cingulate cortical neurons with intact thalamic inputs.Eur J Neurosci. 2007 May;25(9):2847-61. doi: 10.1111/j.1460-9568.2007.05485.x. Eur J Neurosci. 2007. PMID: 17561847
-
Control of Homeostatic Synaptic Plasticity by AKAP-Anchored Kinase and Phosphatase Regulation of Ca2+-Permeable AMPA Receptors.J Neurosci. 2018 Mar 14;38(11):2863-2876. doi: 10.1523/JNEUROSCI.2362-17.2018. Epub 2018 Feb 13. J Neurosci. 2018. PMID: 29440558 Free PMC article.
-
[The role of cluster receptor state in synaptic plasticity].Zh Vyssh Nerv Deiat Im I P Pavlova. 2008 May-Jun;58(3):276-93. Zh Vyssh Nerv Deiat Im I P Pavlova. 2008. PMID: 18689239 Review. Russian.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
Cited by
-
Contribution of S4 segments and S4-S5 linkers to the low-voltage activation properties of T-type CaV3.3 channels.PLoS One. 2018 Feb 23;13(2):e0193490. doi: 10.1371/journal.pone.0193490. eCollection 2018. PLoS One. 2018. PMID: 29474447 Free PMC article.
-
Sleep slow wave-related homo and heterosynaptic LTD of intrathalamic GABAAergic synapses: involvement of T-type Ca2+ channels and metabotropic glutamate receptors.J Neurosci. 2015 Jan 7;35(1):64-73. doi: 10.1523/JNEUROSCI.2748-14.2015. J Neurosci. 2015. PMID: 25568103 Free PMC article.
-
Maturational Stage-Dependent Contributions of the Cav3.2 T-Type Calcium Channel to Dentate Gyrus Granule Cell Excitability.eNeuro. 2025 Apr 4;12(4):ENEURO.0423-24.2025. doi: 10.1523/ENEURO.0423-24.2025. Print 2025 Apr. eNeuro. 2025. PMID: 40068874 Free PMC article.
-
A novel mechanism for short-term post-tetanic plasticity in thalamocortical neurons.Brain Res. 2025 Jul 15;1859:149654. doi: 10.1016/j.brainres.2025.149654. Epub 2025 Apr 21. Brain Res. 2025. PMID: 40268039
-
Long-term effects of a double hit murine model for schizophrenia on parvalbumin expressing cells and plasticity-related molecules in the thalamic reticular nucleus and the habenula.Transl Psychiatry. 2024 Oct 24;14(1):450. doi: 10.1038/s41398-024-03166-6. Transl Psychiatry. 2024. PMID: 39448557 Free PMC article.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. - PubMed
-
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
