LTP-induced long-term stabilization of individual nascent dendritic spines
- PMID: 23303946
- PMCID: PMC6704923
- DOI: 10.1523/JNEUROSCI.1404-12.2013
LTP-induced long-term stabilization of individual nascent dendritic spines
Abstract
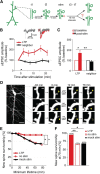
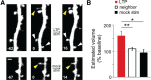
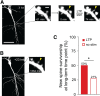
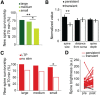
Learning new tasks has been associated with increased growth and stabilization of new dendritic spines. We examined whether long-term potentiation (LTP), a key cellular mechanism thought to underlie learning, plays a role in selective stabilization of individual new spines during circuit plasticity. Using two-photon glutamate uncaging, we stimulated nascent spines on dendrites of rat hippocampal CA1 neurons with patterns that induce LTP and then monitored spine survival rates using time-lapse imaging. Remarkably, we found that LTP-inducing stimuli increased the long-term survivorship (>14 h) of individual new spines. Activity-induced new spine stabilization required NMDA receptor activation and was specific for stimuli that induced LTP. Moreover, abrogating CaMKII binding to the NMDA receptor abolished activity-induced new spine stabilization. Our findings demonstrate for the first time that, in addition to enhancing the efficacy of preexisting synapses, LTP-inducing stimuli promote the transition of nascent spines from a short-lived, transient state to a longer-lived, persistent state.
Figures


Similar articles
-
Activity-Dependent Stabilization of Nascent Dendritic Spines Requires Nonenzymatic CaMKIIα Function.J Neurosci. 2024 Jan 10;44(2):e1393222023. doi: 10.1523/JNEUROSCI.1393-22.2023. J Neurosci. 2024. PMID: 38050081 Free PMC article.
-
Spine expansion and stabilization associated with long-term potentiation.J Neurosci. 2008 May 28;28(22):5740-51. doi: 10.1523/JNEUROSCI.3998-07.2008. J Neurosci. 2008. PMID: 18509035 Free PMC article.
-
Activation of CaMKII in single dendritic spines during long-term potentiation.Nature. 2009 Mar 19;458(7236):299-304. doi: 10.1038/nature07842. Nature. 2009. PMID: 19295602 Free PMC article.
-
Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses.Psychoneuroendocrinology. 2009 Dec;34 Suppl 1:S130-42. doi: 10.1016/j.psyneuen.2009.06.003. Psychoneuroendocrinology. 2009. PMID: 19596521 Free PMC article. Review.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
Cited by
-
Heterosynaptic structural plasticity on local dendritic segments of hippocampal CA1 neurons.Cell Rep. 2015 Jan 13;10(2):162-9. doi: 10.1016/j.celrep.2014.12.016. Epub 2014 Dec 31. Cell Rep. 2015. PMID: 25558061 Free PMC article.
-
Serotonin modulates excitatory synapse maturation in the developing prefrontal cortex.Nat Commun. 2024 Feb 16;15(1):1368. doi: 10.1038/s41467-024-45734-w. Nat Commun. 2024. PMID: 38365905 Free PMC article.
-
MCU expression in hippocampal CA2 neurons modulates dendritic mitochondrial morphology and synaptic plasticity.Sci Rep. 2025 Feb 6;15(1):4540. doi: 10.1038/s41598-025-85958-4. Sci Rep. 2025. PMID: 39915602 Free PMC article.
-
The fate of hippocampal synapses depends on the sequence of plasticity-inducing events.Elife. 2018 Oct 12;7:e39151. doi: 10.7554/eLife.39151. Elife. 2018. PMID: 30311904 Free PMC article.
-
Activity-Dependent Stabilization of Nascent Dendritic Spines Requires Nonenzymatic CaMKIIα Function.J Neurosci. 2024 Jan 10;44(2):e1393222023. doi: 10.1523/JNEUROSCI.1393-22.2023. J Neurosci. 2024. PMID: 38050081 Free PMC article.
References
-
- Bailey CH, Alberini C, Ghirardi M, Kandel ER. Molecular and structural changes underlying long-term memory storage in Aplysia. Adv Second Messenger Phosphoprotein Res. 1994;29:529–544. - PubMed
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
-
- Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
