Influence of core auditory cortical areas on acoustically evoked activity in contralateral primary auditory cortex
- PMID: 23303954
- PMCID: PMC6704916
- DOI: 10.1523/JNEUROSCI.1784-12.2013
Influence of core auditory cortical areas on acoustically evoked activity in contralateral primary auditory cortex
Abstract
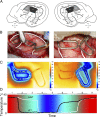
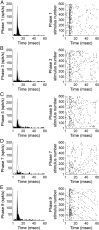
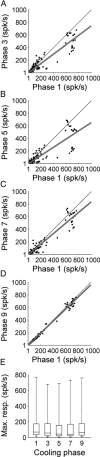
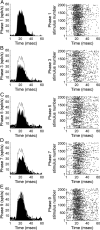
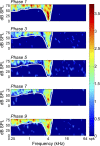
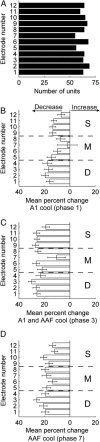
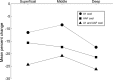
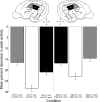
In contrast to numerous studies of transcallosal communication in visual and somatosensory cortices, the functional properties of interhemispheric connections between auditory cortical fields have not been widely scrutinized. Therefore, the purpose of the present investigation was to measure the magnitude and type (inhibitory/excitatory) of modulatory properties of core auditory fields on contralateral primary auditory cortex (A1) activity. We combined single-unit neuronal recordings with reversible cooling deactivation techniques to measure variations in contralateral A1 response levels during A1, anterior auditory field (AAF), or simultaneous A1 and AAF neuronal discharge suppression epochs in cat auditory cortex. Cortical activity was evoked by presentation of pure tones, noise bursts, and frequency-modulated (FM) sweeps before, during, and after cortical deactivation periods. Comparisons of neuronal response changes before and during neuronal silencing revealed three major findings. First, deactivation of A1 and AAF-induced significant peak response reductions in contralateral A1 activity during simple (tonal) and complex (noise bursts and FM sweeps) acoustic exposure. Second, decreases in A1 neuronal activity appear to be in agreement with anatomical laminar termination patterns emanating from contralateral auditory cortex fields. Third, modulatory properties of core auditory areas lack hemispheric lateralization. These findings demonstrate that during periods of acoustic exposure, callosal projections emanating from core auditory areas modulate A1 neuronal activity via excitatory inputs.
Figures





Similar articles
-
Effects of core auditory cortex deactivation on neuronal response to simple and complex acoustic signals in the contralateral anterior auditory field.Cereb Cortex. 2015 Jan;25(1):84-96. doi: 10.1093/cercor/bht205. Epub 2013 Aug 19. Cereb Cortex. 2015. PMID: 23960202
-
Differential modulatory influences between primary auditory cortex and the anterior auditory field.J Neurosci. 2009 Jul 1;29(26):8350-62. doi: 10.1523/JNEUROSCI.6001-08.2009. J Neurosci. 2009. PMID: 19571126 Free PMC article.
-
Evidence for hierarchical processing in cat auditory cortex: nonreciprocal influence of primary auditory cortex on the posterior auditory field.J Neurosci. 2009 Nov 11;29(45):14323-33. doi: 10.1523/JNEUROSCI.2905-09.2009. J Neurosci. 2009. PMID: 19906979 Free PMC article.
-
Auditory corticocortical interconnections in the cat: evidence for parallel and hierarchical arrangement of the auditory cortical areas.Exp Brain Res. 1991;86(3):483-505. doi: 10.1007/BF00230523. Exp Brain Res. 1991. PMID: 1722171
-
Effect of age on lateralized auditory processing.Hear Res. 2023 Jul;434:108791. doi: 10.1016/j.heares.2023.108791. Epub 2023 May 11. Hear Res. 2023. PMID: 37209509 Review.
Cited by
-
Moderate Cortical Cooling Eliminates Thalamocortical Silent States during Slow Oscillation.J Neurosci. 2015 Sep 23;35(38):13006-19. doi: 10.1523/JNEUROSCI.1359-15.2015. J Neurosci. 2015. PMID: 26400932 Free PMC article.
-
Selective Interruption of Auditory Interhemispheric Cross Talk Impairs Discrimination Learning of Frequency-Modulated Tone Direction But Not Gap Detection and Discrimination.J Neurosci. 2022 Mar 9;42(10):2025-2038. doi: 10.1523/JNEUROSCI.0216-21.2022. Epub 2022 Jan 21. J Neurosci. 2022. PMID: 35064004 Free PMC article.
-
Posterior Inferotemporal Cortex Cells Use Multiple Input Pathways for Shape Encoding.J Neurosci. 2017 May 10;37(19):5019-5034. doi: 10.1523/JNEUROSCI.2674-16.2017. Epub 2017 Apr 17. J Neurosci. 2017. PMID: 28416597 Free PMC article.
-
Cortical hemispheric asymmetries are present at young ages and further develop into adolescence.Hum Brain Mapp. 2018 Feb;39(2):941-954. doi: 10.1002/hbm.23893. Epub 2017 Nov 13. Hum Brain Mapp. 2018. PMID: 29134751 Free PMC article.
-
Interhemispheric Callosal Projections Sharpen Frequency Tuning and Enforce Response Fidelity in Primary Auditory Cortex.eNeuro. 2020 Aug 17;7(4):ENEURO.0256-20.2020. doi: 10.1523/ENEURO.0256-20.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32769158 Free PMC article.
References
-
- Abel PL, O'Brien BJ, Olavarria JF. Organization of callosal linkages in visual area V2 of macaque monkey. J Comp Neurol. 2000;428:278–293. - PubMed
-
- Adams JC, Warr WB. Origins of axons in the cat's acoustic striae determined by injection of horseradish peroxidase into severed tracts. J Comp Neurol. 1976;170:107–121. - PubMed
-
- Adey WR. Biophysical and metabolic bases of cooling effects on cortical membrane potentials in the cat. Exp Neurol. 1974;42:113–140. - PubMed
-
- Aitkin LM, Kudo M, Irvine DR. Connections of the primary auditory cortex in the common marmoset, Callithrix jacchus jacchus. J Comp Neurol. 1988;269:235–248. - PubMed
-
- Alibardi L. Cytology, synaptology and immunocytochemistry of commissural neurons and their putative axonal terminals in the dorsal cochlear nucleus of the rat. Ann Anat. 2000;182:207–220. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
