Rational design of vaccines for AIDS and influenza
- PMID: 23303965
- PMCID: PMC3540641
Rational design of vaccines for AIDS and influenza
Abstract
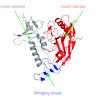
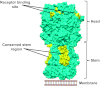
Successful immunization against challenging infectious diseases requires novel approaches to vaccine design. To control diseases of high human health impact such as AIDS and influenza by vaccination requires an understanding of the mechanisms of viral entry and identification of highly conserved vulnerable regions of the virus to which immune responses are not normally directed. Structural biology has provided important information about the three-dimensional organization and chemical structure of the HIV-1 and influenza glycoproteins. By harnessing structural biology, monoclonal antibody specificity, genomics, and informatics, we have been able to define neutralizing antibodies of exceptional breadth and potency against circulating strains of HIV-1, and we have begun to develop immunogens to elicit such antibodies. Similarly, for influenza, understanding of this target has led to structural and genetic approaches to the development of new immunogens that provide a proof of concept for universal influenza vaccination.
Conflict of interest statement
Potential Conflicts of Interest: None disclosed.
Figures

Similar articles
-
The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus.Nat Rev Microbiol. 2008 Feb;6(2):143-55. doi: 10.1038/nrmicro1819. Nat Rev Microbiol. 2008. PMID: 18197170 Review.
-
Mapping of a Novel H3-Specific Broadly Neutralizing Monoclonal Antibody Targeting the Hemagglutinin Globular Head Isolated from an Elite Influenza Virus-Immunized Donor Exhibiting Serological Breadth.J Virol. 2020 Feb 28;94(6):e01035-19. doi: 10.1128/JVI.01035-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31826999 Free PMC article.
-
Vaccine outlooks. After negative publicity and a series of setbacks over HIV/AIDS and influenza, the prospects for research on new vaccines are improving.EMBO Rep. 2010 Oct;11(10):738-41. doi: 10.1038/embor.2010.136. EMBO Rep. 2010. PMID: 20877293 Free PMC article.
-
Call for a paradigm shift in the design of universal influenza vaccines by harnessing multiple correlates of protection.Expert Opin Drug Discov. 2020 Dec;15(12):1441-1455. doi: 10.1080/17460441.2020.1801629. Epub 2020 Aug 12. Expert Opin Drug Discov. 2020. PMID: 32783765 Review.
-
Broadly Neutralizing Antibodies to Highly Antigenically Variable Viruses as Templates for Vaccine Design.Curr Top Microbiol Immunol. 2020;428:31-87. doi: 10.1007/82_2020_221. Curr Top Microbiol Immunol. 2020. PMID: 32648034 Review.
Cited by
-
Challenges to the development of vaccines to hepatitis C virus that elicit neutralizing antibodies.Front Microbiol. 2014 Jul 3;5:329. doi: 10.3389/fmicb.2014.00329. eCollection 2014. Front Microbiol. 2014. PMID: 25071742 Free PMC article. Review.
-
Hepatitis C Virus E2 Envelope Glycoprotein Induces an Immunoregulatory Phenotype in Macrophages.Hepatology. 2019 May;69(5):1873-1884. doi: 10.1002/hep.29843. Epub 2018 May 24. Hepatology. 2019. PMID: 29443378 Free PMC article.
-
Broadly neutralizing influenza hemagglutinin stem-specific antibody CR8020 targets residues that are prone to escape due to host selection pressure.Cell Host Microbe. 2014 May 14;15(5):644-51. doi: 10.1016/j.chom.2014.04.009. Cell Host Microbe. 2014. PMID: 24832457 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
