A new stem cell biology: the continuum and microvesicles
- PMID: 23303982
- PMCID: PMC3540600
A new stem cell biology: the continuum and microvesicles
Abstract
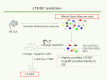
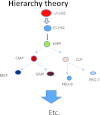
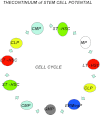
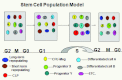
The hierarchical models of stem cell biology have been based on work first demonstrating pluripotental spleen-colony-forming units, then showing progenitors with many differentiation fates assayed in in vitro culture; there followed the definition and separation of "stem cells" using monoclonal antibodies to surface epitopes and fluorescent-activated cell characterization and sorting (FACS). These studies led to an elegant model of stem cell biology in which primitive dormant G0 stem cells with tremendous proliferative and differentiative potential gave rise to progressively more restricted and differentiated classes of stem/progenitor cells, and finally differentiated marrow hematopoietic cells. The holy grail of hematopoietic stem cell biology became the purification of the stem cell and the clonal definition of this cell. Most recently, the long-term repopulating hematopoietic stem cell (LT-HSC) has been believed to be a lineage negative sca-1+C-kit+ Flk3- and CD150+ cell. However, a series of studies over the past 10 years has indicated that murine marrow stem cells continuously change phenotype with cell cycle passage. We present here studies using tritiated thymidine suicide and pyronin-Hoechst FACS separations indicating that the murine hematopoietic stem cell is a cycling cell. This would indicate that the hematopoietic stem cell must be continuously changing in phenotype and, thus, could not be purified. The extant data indicate that murine marrow stem cells are continually transiting cell cycle and that the purification has discarded these cycling cells. Further in vivo BrdU studies indicate that the "quiescent" LT-HSC in G0 rapidly transits cycle. Further complexity of the marrow stem cell system is indicated by studies on cell-derived microvesicles showing that they enter marrow cells and transcriptionally alter their cell fate and phenotype. Thus, the stem cell model is a model of continuing changing potential tied to cell cycle and microvesicle exposure. The challenge of the future is to define the stem cell population, not purify the stem cell. We are at the beginning of elucidation of quantum stemomics.
Conflict of interest statement
Potential Conflicts of Interest: None disclosed.
Figures






Similar articles
-
Marrow Hematopoietic Stem Cells Revisited: They Exist in a Continuum and are Not Defined by Standard Purification Approaches; Then There are the Microvesicles.Front Oncol. 2014 Apr 4;4:56. doi: 10.3389/fonc.2014.00056. eCollection 2014. Front Oncol. 2014. PMID: 24772390 Free PMC article. Review.
-
Differentiation Epitopes Define Hematopoietic Stem Cells and Change with Cell Cycle Passage.Stem Cell Rev Rep. 2022 Oct;18(7):2351-2364. doi: 10.1007/s12015-022-10374-4. Epub 2022 May 3. Stem Cell Rev Rep. 2022. PMID: 35503199 Free PMC article.
-
Phenotypic analysis and isolation of murine hematopoietic stem cells and lineage-committed progenitors.J Vis Exp. 2012 Jul 8;(65):3736. doi: 10.3791/3736. J Vis Exp. 2012. PMID: 22805770 Free PMC article.
-
The murine long-term multi-lineage renewal marrow stem cell is a cycling cell.Leukemia. 2014 Apr;28(4):813-22. doi: 10.1038/leu.2013.252. Epub 2013 Aug 30. Leukemia. 2014. PMID: 23989430
-
The stem cell continuum: cell cycle, injury, and phenotype lability.Ann N Y Acad Sci. 2007 Jun;1106:20-9. doi: 10.1196/annals.1392.016. Epub 2007 Mar 14. Ann N Y Acad Sci. 2007. PMID: 17360803 Review.
Cited by
-
Matrix-bound nanovesicles within ECM bioscaffolds.Sci Adv. 2016 Jun 10;2(6):e1600502. doi: 10.1126/sciadv.1600502. eCollection 2016 Jun. Sci Adv. 2016. PMID: 27386584 Free PMC article.
-
Porcine Small Intestinal Submucosa Alters the Biochemical Properties of Wound Healing: A Narrative Review.Biomedicines. 2022 Sep 7;10(9):2213. doi: 10.3390/biomedicines10092213. Biomedicines. 2022. PMID: 36140314 Free PMC article. Review.
-
Extracellular Vesicles: Decoding a New Language for Cellular Communication in Early Embryonic Development.Front Cell Dev Biol. 2018 Aug 28;6:94. doi: 10.3389/fcell.2018.00094. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30211159 Free PMC article. Review.
-
Chronic myeloid leukemia stem cells.Leukemia. 2019 Jul;33(7):1543-1556. doi: 10.1038/s41375-019-0490-0. Epub 2019 May 24. Leukemia. 2019. PMID: 31127148 Free PMC article. Review.
-
Human and murine very small embryonic-like cells represent multipotent tissue progenitors, in vitro and in vivo.Stem Cells Dev. 2014 Apr 1;23(7):689-701. doi: 10.1089/scd.2013.0362. Epub 2014 Feb 4. Stem Cells Dev. 2014. PMID: 24372153 Free PMC article.
References
-
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–22. - PubMed
-
- Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287–99. - PubMed
-
- Pluznik DH, Sachs L. The cloning of normal “mast” cells in tissue culture. J Cell Physiol. 1965;66:319–24. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
