A "novel" protocol for the analysis of hydroxycinnamic acids in leaf tissue of chicory (Cichorium intybus L., Asteraceae)
- PMID: 23304076
- PMCID: PMC3523586
- DOI: 10.1100/2012/142983
A "novel" protocol for the analysis of hydroxycinnamic acids in leaf tissue of chicory (Cichorium intybus L., Asteraceae)
Abstract
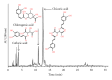
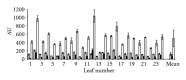
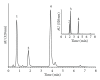
A "novel" protocol is presented for easy and reliable estimation of soluble hydroxycinnamate levels in Cichorium intybus L. leaf tissue in large-scale experiments. Samples were standardized by punching 6 discs per leaf, and hydroxycinnamates were extracted by submerging the discs in 80% ethanol with 5% acetic acid for at least 48 h in the darkness at 4°C. Residual dry mass of the discs was used for a posteriori correction of compound levels. Chlorophyll was eliminated by chloroform, and the aqueous phases were transferred to microplates, dried, and dissolved in 50% methanol for HPLC analysis and storage. An HPLC program of 8 min was developed for the analysis of the extracts. Comparisons with extractions of liquid nitrogen powders indicated that the novel extraction method was reliable. No degradation of the major hydroxycinnamates-caftaric, chlorogenic, and chicoric acids-was observed, during maceration at ambient temperatures, or after storage for 1 year.
Figures
References
-
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. American Journal of Clinical Nutrition. 2004;79(5):727–747. - PubMed
-
- Shahidi F, Chandrasekara A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochemistry Reviews. 2010;9(1):147–170.
-
- Cheng JC, Dai F, Zhou B, Yang L, Liu ZL. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: mechanism and structure-activity relationship. Food Chemistry. 2007;104(1):132–139.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

