Expression Pattern of Peroxisome Proliferator-Activated Receptors in Rat Hippocampus following Cerebral Ischemia and Reperfusion Injury
- PMID: 23304113
- PMCID: PMC3523613
- DOI: 10.1155/2012/596394
Expression Pattern of Peroxisome Proliferator-Activated Receptors in Rat Hippocampus following Cerebral Ischemia and Reperfusion Injury
Abstract
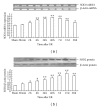
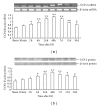
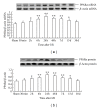
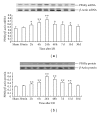
The present study was designed to investigate the pattern of time-dependent expression of peroxisome proliferator-activated receptors (PPARα, β, and γ) after global cerebral ischemia and reperfusion (I/R) damage in the rat hippocampus. Male Sprague Dawley (SD) rats were subjected to global cerebral I/R. The rat hippocampi were isolated to detect the expression of PPARs mRNA and protein levels at 30 min-30 d after I/R by RT-PCR and Western blot analysis, respectively. The expression levels of PPARs mRNA and protein in the rat hippocampus significantly increased and peaked at 24 h for PPARα and γ (at 48 h for PPARβ) after I/R, then gradually decreased, and finally approached control levels on d 30. The present results suggest that global cerebral I/R can cause obvious increases of hippocampal PPARs mRNA and protein expression within 15 d after I/R. These findings may help to guide the experimental and clinical therapeutic use of PPARs agonists against brain injury.
Figures


References
-
- Liang HW, Qiu SF, Shen J, et al. Genistein attenuates oxidative stress and neuronal damage following transient global cerebral ischemia in rat hippocampus. Neuroscience Letters. 2008;438(1):116–120. - PubMed
-
- Büttnera F, Cordesb C, Gerlachc F, et al. Genomic response of the rat brain to global ischemia and reperfusion. Brain Research. 2009;1252:1–14. - PubMed
-
- Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor α, β, and γ and the retinoid X receptor α, β, and γ in rat central nervous system. Journal of Neurochemistry. 1998;70(4):1366–1375. - PubMed
-
- Moreno S, Farioli-vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123(1):131–145. - PubMed
LinkOut - more resources
Full Text Sources

