Chlorella sorokiniana-Induced Activation and Maturation of Human Monocyte-Derived Dendritic Cells through NF-κB and PI3K/MAPK Pathways
- PMID: 23304212
- PMCID: PMC3523612
- DOI: 10.1155/2012/735396
Chlorella sorokiniana-Induced Activation and Maturation of Human Monocyte-Derived Dendritic Cells through NF-κB and PI3K/MAPK Pathways
Abstract
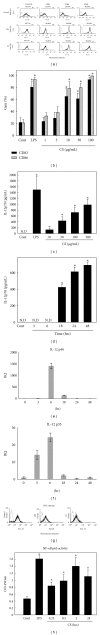
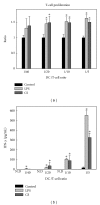
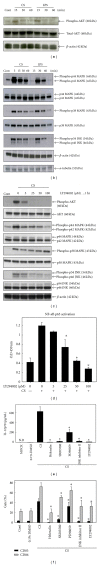
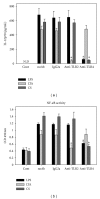
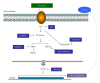
Chlorella sorokiniana (CS) is a unicellular green alga. The extracts of Chlorella have been used as treatments for relieving hypertension and modulating immune response. The detailed mechanisms are not clear yet. In this study, we sought to study the molecular mechanisms for the polysaccharide fraction of CS-induced immune response. We pulsed dendritic cells (DCs) with CS and found that CS could maturate DCs. CS-maturated DC could activate naïve T cells and stimulate T-cell proliferation and IFN-γ secretion. Furthermore, CS activated PI3K and MAPKs signaling pathways in DCs by interacting with TLR4 receptor. These CS-activated signaling pathways could further activate NF-κB and induce IL-12 production in DCs. This study provides molecular mechanisms for CS-induced DCs activation and immune response.
Figures
Similar articles
-
An immunomodulatory protein, Ling Zhi-8, induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and MAPK pathways.J Leukoc Biol. 2009 Oct;86(4):877-89. doi: 10.1189/jlb.0708441. Epub 2009 Jun 4. J Leukoc Biol. 2009. PMID: 19498044
-
Threonyl-tRNA Synthetase Promotes T Helper Type 1 Cell Responses by Inducing Dendritic Cell Maturation and IL-12 Production via an NF-κB Pathway.Front Immunol. 2020 Oct 14;11:571959. doi: 10.3389/fimmu.2020.571959. eCollection 2020. Front Immunol. 2020. PMID: 33178197 Free PMC article.
-
PPAR-gamma agonist inhibits Ang II-induced activation of dendritic cells via the MAPK and NF-kappaB pathways.Immunol Cell Biol. 2010 Mar-Apr;88(3):305-12. doi: 10.1038/icb.2009.100. Epub 2009 Dec 8. Immunol Cell Biol. 2010. PMID: 19997078
-
Lysyl-Transfer RNA Synthetase Induces the Maturation of Dendritic Cells through MAPK and NF-κB Pathways, Strongly Contributing to Enhanced Th1 Cell Responses.J Immunol. 2018 Nov 1;201(9):2832-2841. doi: 10.4049/jimmunol.1800386. Epub 2018 Oct 1. J Immunol. 2018. PMID: 30275047
-
Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement.J Dermatol Sci. 2006 Apr;42(1):1-11. doi: 10.1016/j.jdermsci.2005.11.004. Epub 2005 Dec 13. J Dermatol Sci. 2006. PMID: 16352421 Review.
Cited by
-
Extracts from Microalga Chlorella sorokiniana Exert an Anti-Proliferative Effect and Modulate Cytokines in Sheep Peripheral Blood Mononuclear Cells.Animals (Basel). 2019 Jan 30;9(2):45. doi: 10.3390/ani9020045. Animals (Basel). 2019. PMID: 30704147 Free PMC article.
-
A Water Extract from Chlorella sorokiniana Cell Walls Stimulates Growth of Bone Marrow Cells and Splenocytes.Nutrients. 2022 Jul 15;14(14):2901. doi: 10.3390/nu14142901. Nutrients. 2022. PMID: 35889858 Free PMC article.
-
Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo.BMC Complement Altern Med. 2017 Feb 1;17(1):88. doi: 10.1186/s12906-017-1611-9. BMC Complement Altern Med. 2017. PMID: 28143460 Free PMC article.
-
Intracellular signaling pathway in dendritic cells and antigen transport pathway in vivo mediated by an OVA@DDAB/PLGA nano-vaccine.J Nanobiotechnology. 2021 Nov 27;19(1):394. doi: 10.1186/s12951-021-01116-8. J Nanobiotechnology. 2021. PMID: 34838057 Free PMC article.
-
Microalgae: A Promising Source of Valuable Bioproducts.Biomolecules. 2020 Aug 6;10(8):1153. doi: 10.3390/biom10081153. Biomolecules. 2020. PMID: 32781745 Free PMC article. Review.
References
-
- Merchant RE, Andre CA. A review of recent clinical trials of the nutritional supplement Chlorella pyrenoidosa in the treatment of fibromyalgia, hypertension, and ulcerative colitis. Alternative Therapies in Health and Medicine. 2001;7(3):79–91. - PubMed
-
- Yang F, Shi Y, Sheng J, Hu Q. In vivo immunomodulatory activity of polysaccharides derived from Chlorella pyrenoidosa . European Food Research and Technology. 2006;224(2):225–228.
-
- An HJ, Rim HK, Jeong HJ, Hong SH, Um JY, Kim HM. Hot water extracts of Chlorella vulgaris improve immune function in protein-deficient weanling mice and immune cells. Immunopharmacology and Immunotoxicology. 2010;32(4):585–592. - PubMed
-
- Lin YL, Liang YC, Lee SS, Chiang BL. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and p38 mitogen-activated protein kinase pathways. Journal of Leukocyte Biology. 2005;78(2):533–543. - PubMed
LinkOut - more resources
Full Text Sources

