Aqueous Extract of Solanum nigrum Leaf Activates Autophagic Cell Death and Enhances Docetaxel-Induced Cytotoxicity in Human Endometrial Carcinoma Cells
- PMID: 23304219
- PMCID: PMC3526186
- DOI: 10.1155/2012/859185
Aqueous Extract of Solanum nigrum Leaf Activates Autophagic Cell Death and Enhances Docetaxel-Induced Cytotoxicity in Human Endometrial Carcinoma Cells
Abstract
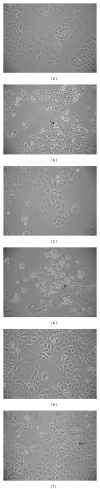
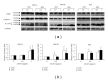
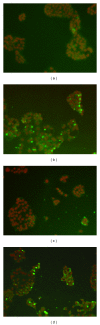
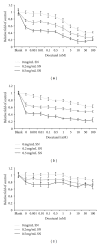
Chemotherapy is the main approach in dealing with advanced and recurrent endometrial cancer. An effective complementary ingredient can be helpful in improving the clinical outcome. Aqueous extract of Solanum nigrum leaf (AE-SN) is a principal ingredient for treating cancer patients in traditional Chinese medicinal practice but lacks sufficient evidence to verify its tumor suppression efficacy. This study evaluated the antitumor effects of AE-SN and also assessed the synergistic effects of AE-SN with docetaxel On the human endometrial cancer cell lines, HEC1A, HEC1B, and KLE. The activation of apoptotic markers, caspase-3 and poly-ADP-ribose polymerase, and autophagic marker, microtubule-associated protein 1 light chain 3 A/B, wAS determined to clarify the cell death pathways responsible for AE-SN induced tumor cell death. Results indicated that AE-SN-treatment has significant cytotoxicity on the tested endometrial cancer cells with accumulation of LC3 A/B II and demonstrated a synergistic effect of AE-SN and docetaxel in HEC1A and HEC1B cells, but not KLE cells. In conclusion, AE-SN treatment was effective in suppressing endometrial cancer cells via the autophagic pathway and was also capable of enhancing the cytotoxicity of docetaxel in human endometrial cancer cells. Our results provide meaningful evidence for integrative cancer therapy in the future.
Figures

Similar articles
-
Antitumor Effects and Biological Mechanism of Action of the Aqueous Extract of the Camptotheca acuminata Fruit in Human Endometrial Carcinoma Cells.Evid Based Complement Alternat Med. 2014;2014:564810. doi: 10.1155/2014/564810. Epub 2014 May 21. Evid Based Complement Alternat Med. 2014. PMID: 24963324 Free PMC article.
-
Cisplatin-, Doxorubicin-, and Docetaxel-Induced Cell Death Promoted by the Aqueous Extract of Solanum nigrum in Human Ovarian Carcinoma Cells.Integr Cancer Ther. 2015 Nov;14(6):546-55. doi: 10.1177/1534735415588826. Epub 2015 Jun 11. Integr Cancer Ther. 2015. PMID: 26069278
-
Aqueous Extract of Solanum nigrum Leaves Induces Autophagy and Enhances Cytotoxicity of Cisplatin, Doxorubicin, Docetaxel, and 5-Fluorouracil in Human Colorectal Carcinoma Cells.Evid Based Complement Alternat Med. 2013;2013:514719. doi: 10.1155/2013/514719. Epub 2013 Jun 17. Evid Based Complement Alternat Med. 2013. PMID: 23843876 Free PMC article.
-
Integrated Treatment of Aqueous Extract of Solanum nigrum-Potentiated Cisplatin- and Doxorubicin-Induced Cytotoxicity in Human Hepatocellular Carcinoma Cells.Evid Based Complement Alternat Med. 2015;2015:675270. doi: 10.1155/2015/675270. Epub 2015 Jun 28. Evid Based Complement Alternat Med. 2015. PMID: 26221175 Free PMC article.
-
Current development in integrative therapy of traditional Chinese medicine for cancer treatment: A mini-review.J Tradit Complement Med. 2019 Jul 9;10(5):429-433. doi: 10.1016/j.jtcme.2019.07.001. eCollection 2020 Sep. J Tradit Complement Med. 2019. PMID: 32953557 Free PMC article. Review.
Cited by
-
Antioxidant Activities of Solanum Nigrum L. Leaf Extracts Determined in in vitro Cellular Models.Foods. 2019 Feb 8;8(2):63. doi: 10.3390/foods8020063. Foods. 2019. PMID: 30744041 Free PMC article.
-
Inhibition of aqueous extracts of Solanum nigrum (AESN) on oral cancer through regulation of mitochondrial fission.J Tradit Complement Med. 2017 Jun 20;8(1):220-225. doi: 10.1016/j.jtcme.2017.05.011. eCollection 2018 Jan. J Tradit Complement Med. 2017. PMID: 29322012 Free PMC article.
-
Solanine Attenuates Hepatocarcinoma Migration and Invasion Induced by Acetylcholine.Integr Cancer Ther. 2020 Jan-Dec;19:1534735420909895. doi: 10.1177/1534735420909895. Integr Cancer Ther. 2020. PMID: 32975458 Free PMC article.
-
Recent Advances in Anticancer Activity of Novel Plant Extracts and Compounds from Curcuma longa in Hepatocellular Carcinoma.J Gastrointest Cancer. 2023 Jun;54(2):368-390. doi: 10.1007/s12029-022-00809-z. Epub 2022 Mar 14. J Gastrointest Cancer. 2023. PMID: 35285010 Free PMC article. Review.
-
Antitumor Effects and Biological Mechanism of Action of the Aqueous Extract of the Camptotheca acuminata Fruit in Human Endometrial Carcinoma Cells.Evid Based Complement Alternat Med. 2014;2014:564810. doi: 10.1155/2014/564810. Epub 2014 May 21. Evid Based Complement Alternat Med. 2014. PMID: 24963324 Free PMC article.
References
-
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. The Lancet. 2005;366(9484):491–505. - PubMed
-
- Bray F, Loos AH, Oostindier M, Weiderpass E. Geographic and temporal variations in cancer of the corpus uteri: incidence and mortality in pre- and postmenopausal women in Europe. International Journal of Cancer. 2005;117(1):123–131. - PubMed
-
- Creasman W, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. International Journal of Gynecology and Obstetrics. 2006;95(supplement 1):S105–S143. - PubMed
-
- Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a gynecologic oncology group study. Journal of Clinical Oncology. 2006;24(1):36–44. - PubMed
-
- Aalders JG, Abeler V, Kolstad P. Recurrent adenocarcinoma of the endometrium: a clinical and histopathological study of 379 patients. Gynecologic Oncology. 1984;17(1):85–103. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

