Cardiac ion channelopathies and the sudden infant death syndrome
- PMID: 23304551
- PMCID: PMC3529486
- DOI: 10.5402/2012/846171
Cardiac ion channelopathies and the sudden infant death syndrome
Abstract
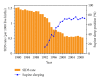
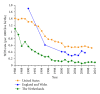
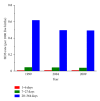
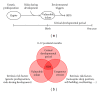
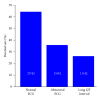
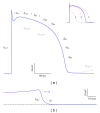
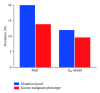
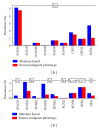
The sudden infant death syndrome (SIDS) causes the sudden death of an apparently healthy infant, which remains unexplained despite a thorough investigation, including the performance of a complete autopsy. The triple risk model for the pathogenesis of SIDS points to the coincidence of a vulnerable infant, a critical developmental period, and an exogenous stressor. Primary electrical diseases of the heart, which may cause lethal arrhythmias as a result of dysfunctioning cardiac ion channels ("cardiac ion channelopathies") and are not detectable during a standard postmortem examination, may create the vulnerable infant and thus contribute to SIDS. Evidence comes from clinical correlations between the long QT syndrome and SIDS as well as genetic analyses in cohorts of SIDS victims ("molecular autopsy"), which have revealed a large number of mutations in ion channel-related genes linked to inheritable arrhythmogenic syndromes, in particular the long QT syndrome, the short QT syndrome, the Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia. Combining data from population-based cohort studies, it can be concluded that at least one out of five SIDS victims carries a mutation in a cardiac ion channel-related gene and that the majority of these mutations are of a known malignant phenotype.
Figures
References
-
- Beckwith JB. Discussion of terminology and definition of sudden infant death syndrome. In: Bergman AB, Beckwith JB, Ray CG, editors. Proceedings of the 2nd International Conference on Causes of Sudden Death in Infants; Seattle, Wash, USA. University of Washington Press; 1970. pp. 14–22.
-
- Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatric Pathology. 1991;11(5):677–684. - PubMed
-
- Krous HF, Beckwith JB, Byard RW, et al. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114(1):234–238. - PubMed
-
- Krous HF. Sudden unexpected death in infancy and the dilemma of defining the sudden infant death syndrome. Current Pediatric Reviews. 2010;6(1):5–12.
-
- Byard RW, Krous HF. Sudden infant death syndrome: overview and update. Pediatric and Developmental Pathology. 2003;6(2):112–127. - PubMed
LinkOut - more resources
Full Text Sources

