Immunotherapy of malignant disease using chimeric antigen receptor engrafted T cells
- PMID: 23304553
- PMCID: PMC3523553
- DOI: 10.5402/2012/278093
Immunotherapy of malignant disease using chimeric antigen receptor engrafted T cells
Abstract
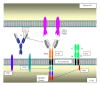
Chimeric antigen receptor- (CAR-) based immunotherapy has been under development for almost 25 years, over which period it has progressed from a new but cumbersome technology to an emerging therapeutic modality for malignant disease. The approach involves the genetic engineering of fusion receptors (CARs) that couple the HLA-independent binding of cell surface target molecules to the delivery of a tailored activating signal to host immune cells. Engineered CARs are delivered most commonly to peripheral blood T cells using a range of vector systems, most commonly integrating viral vectors. Preclinical refinement of this approach has proceeded over several years to the point that clinical testing is now being undertaken at several centres, using increasingly sophisticated and therapeutically successful genetic payloads. This paper considers several aspects of the pre-clinical and clinical development of CAR-based immunotherapy and how this technology is acquiring an increasing niche in the treatment of both solid and haematological malignancies.
Figures
References
-
- Landsberg J, Kohlmeyer J, Renn M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490(7420):412–416. - PubMed
-
- Willemsen RA, Ronteltap C, Chames P, Debets R, Bolhuis RLH. T cell retargeting with MHC class I-restricted antibodies: the CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. Journal of Immunology. 2005;174(12):7853–7858. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

